Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic Lupus Erythematosus (SLE) is a chronic autoimmune disease associated with severe morbidity and mortality. Around 70% of SLE patients follow a relapsing-remitting pattern of disease characterized by unpredictable periods of symptom exacerbation known as flares, followed by periods of disease quiescence. Memory CD4+ T cell subsets have been shown to play an important role in driving the autoantibody production which causes flares in SLE, however the precise T cell changes that accompany flares are unknown.
Methods: CITE-seq was used to assess the transcriptomic profiles of CD4+ memory T cells in flaring and quiescent SLE patients. CD4+ memory T cells were isolated from PBMCs, stained with oligo-conjugated antibodies against surface proteins, and subsequently partitioned, barcoded, and sequenced. We examined samples from 15 distinct patients at two separate clinical visits spaced at least one year apart, yielding 30 paired samples. The longitudinal nature of our data allows us to inspect transcriptional changes both between and within patients.
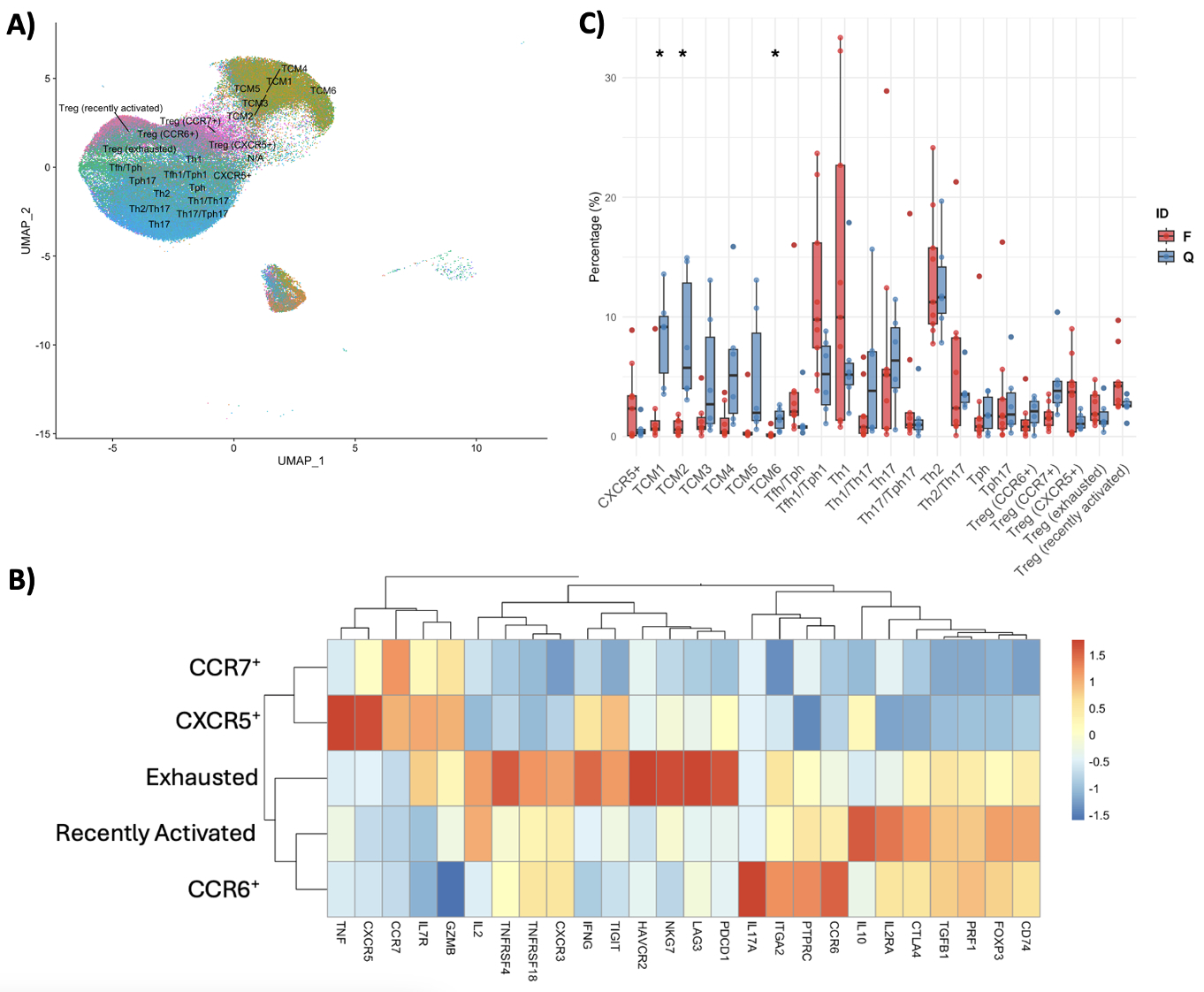
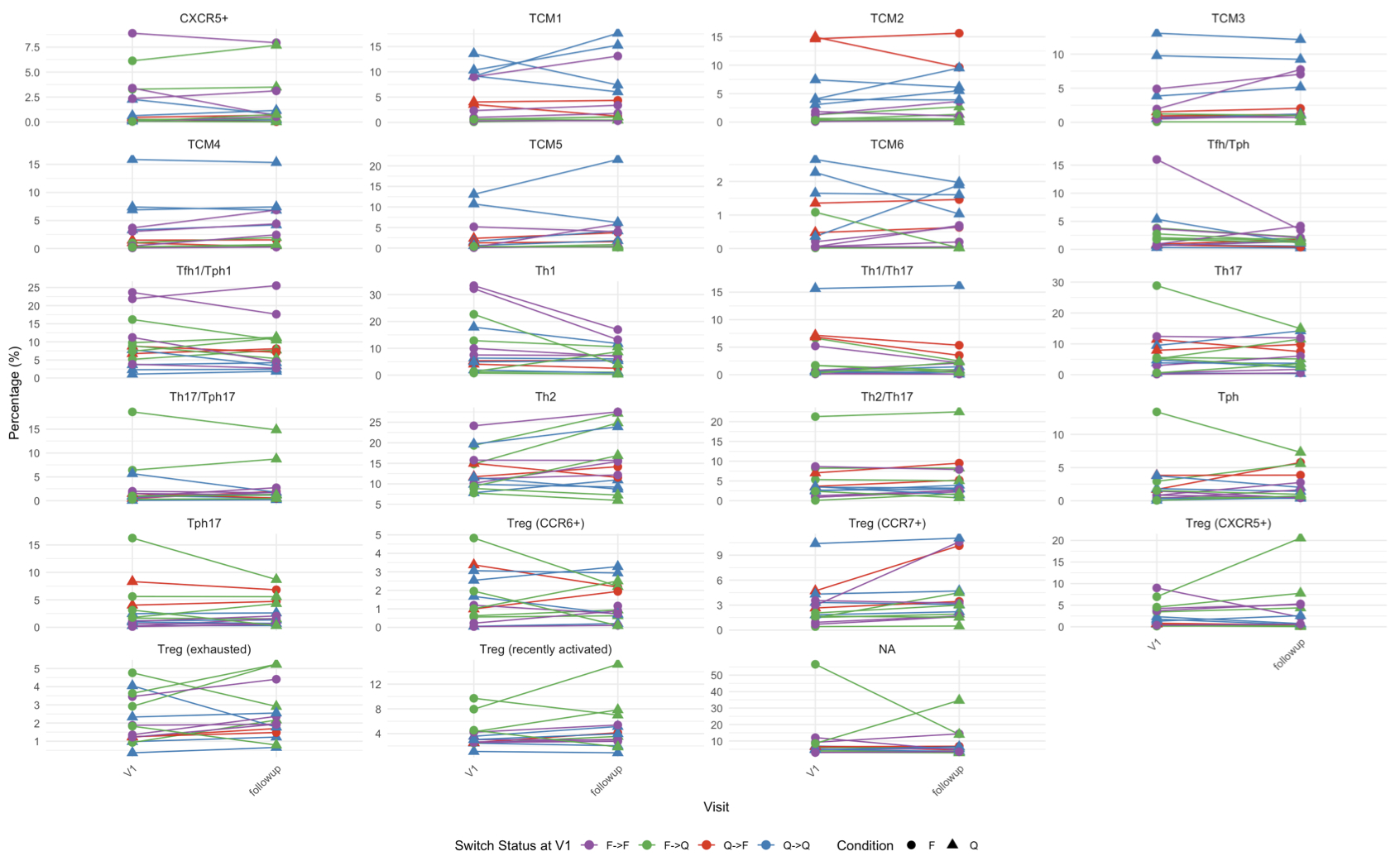
Results: Integration of the gene and surface protein expression data allowed us to identify 23 unique immune populations [Fig.1.A]. Among these, we detected five regulatory T cell (Treg)-enriched clusters expressing canonical Treg-associated genes. Analysis of the relative expression of key molecules among these clusters [Fig.1.B] revealed one subset with high exhaustion marker expression and features consistent with cell reprogramming to a more inflammatory phenotype (increased IFNg and IL-2 expression). Comparing the proportions of this subset between flaring and quiescent patients at baseline [Fig.1.C], we found no differences, suggesting that Treg exhaustion occurs in SLE irrespective of clinical flare status. Although the frequencies were similar, differential gene expression analysis showed significant differences (e.g., IFN-stimulated genes) in flaring and quiescent patients at baseline, including within the exhausted Treg subset. Additionally, samples from quiescent patients were more enriched for central memory T cells (TCMs), specifically TCM1/2/6, compared to flaring patients (p < 0.05) [Fig.1.C]. Conversely, T follicular helper (Tfh), T peripheral helper (Tph), and Th1 cells were more enriched in flaring patients at baseline. Longitudinal analysis demonstrated that TCM proportions remained stable over time [Fig.2], even when patients’ flare status changed between visits.
Conclusion: Treg exhaustion appears consistent regardless of flare status, while differential gene expression highlights the differences between disease states. Quiescent patients show higher enrichment of TCMs, while flaring patients have increased Tfh, Tph, and Th1 cells. These preliminary findings highlight the distinct transcriptional profiles and subset distributions of CD4+ memory T cells in SLE patients, providing insights into the immune mechanisms underlying disease flares and quiescence.
To cite this abstract in AMA style:
Nassar C, Quevedo R, Ciudad M, Faheem Z, Manion K, Munoz-Grajales C, Kim M, Gladman D, Urowitz M, Touma Z, McGaha T, Wither J. Characterizing Memory T Cell Subsets Associated with SLE Etiopathogenesis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/characterizing-memory-t-cell-subsets-associated-with-sle-etiopathogenesis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/characterizing-memory-t-cell-subsets-associated-with-sle-etiopathogenesis/