Session Information
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Juvenile idiopathic arthritis (JIA) is the most common rheumatic disease in children. Tumor necrosis factor inhibitors (TNFi) have demonstrated efficacy and safety in older JIA patients and are approved for use in children over age 2 years. However, use of these therapies in children ≤2 years is not well described despite frequent use in clinical practice. Children ≤2 yo who would benefit from these medications may have delays in treatment due to the lack of FDA approval, resulting in increased morbidity. Our aim was to describe the demographics, medication use, and changes in medication use over time in the cohort of children diagnosed with non-SJIA ≤2 yo enrolled in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry.
Methods: The CARRA registry is a national multicenter observational database for children with rheumatic diseases in the United States. Demographics, disease phenotype, and medication use of patients diagnosed with non-SJIA before the age of 2 years enrolled in the CARRA Registry were analyzed using descriptive statistics. Those who started a TNFi ≤2 yo, >2 yo, and those who never used TNFi were analyzed; summary statistics are presented using median with interquartile range (IQR) for continuous variables and count with frequency for categorical variables. Group comparisons were completed using Welch’s ANOVA for continuous variables and Chi Square test for categorical variables with a significance level of 0.05.
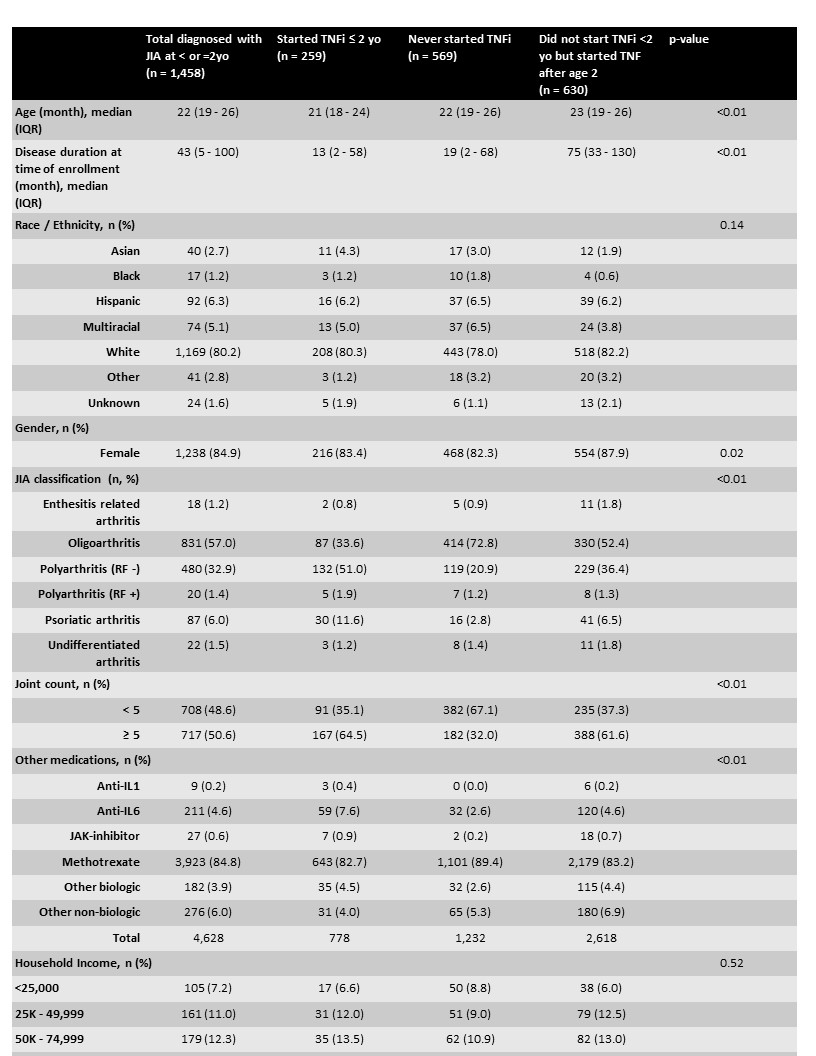
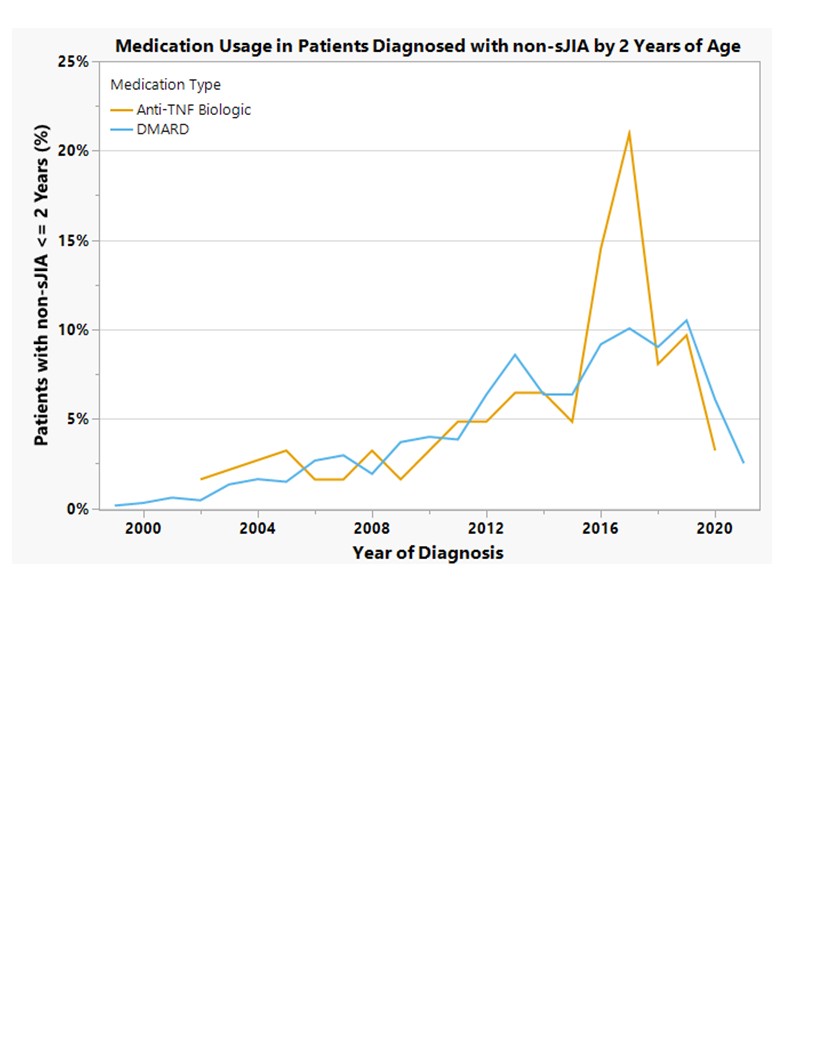
Results: 1,458 patients diagnosed with non-SJIA ≤2 yo were enrolled in the CARRA registry between 2015 and 2021. Consistent with known demographics of JIA subtypes, there were fewer enthesitis related arthritis and RF + polyarticular JIA patients diagnosed before the age of 2. Of these, 259 (18%) started a TNFi before the age of 2. Patients started on TNFi after their 2nd birthday tended to have a longer disease duration at time of registry enrollment as compared to those that started before age 2 (p< 0.01). Early initiation of TNFi did not vary based on race or average income. Half of patients with RF - polyarticular JIA were started on TNFi ≤2 yo, and joint counts of ≥5 had higher rates of early TNFi initiation (Table 1). DMARD and TNFi use before age 2 increased over time but decreased from 2018-2021 (figure 1). Less than 1% of our cohort was started on a non-TNFi biologic at ≤2yo.
Conclusion: Our findings demonstrate that despite the lack of FDA approval, TNFi medications are being utilized in 18% of patients under the age of 2, with higher early utilization rates when olig-JIA patients are removed from the analysis. Possible explanation for decreased use from 2018-2021 could be due to early concerns regarding immunosuppresion in COVID19; future data will clarify. Overall, TNFi medications are an accepted treatment amongst pediatric rheumatologists in early-onset JIA. Future analysis will look at patients diagnosed ≤2yo enrolled in the registry near time of diagnosis, and assess the relationship between disease activity and TNFi initiation, efficacy, and adverse events in this population.
To cite this abstract in AMA style:
Gulla C, Lozy T, Choi D, Janow G, The CARRA Registry Investgators F. Characterization of the Youngest Cohort with Non-Systemic Juvenile Idiopathic Arthritis: Demographics and Medication Use of Patients ≤2 Years of Age in the Childhood Arthritis and Rheumatology Research Alliance Registry [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/characterization-of-the-youngest-cohort-with-non-systemic-juvenile-idiopathic-arthritis-demographics-and-medication-use-of-patients-%e2%89%a42-years-of-age-in-the-childhood-arthritis-and-rheumatology/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/characterization-of-the-youngest-cohort-with-non-systemic-juvenile-idiopathic-arthritis-demographics-and-medication-use-of-patients-%e2%89%a42-years-of-age-in-the-childhood-arthritis-and-rheumatology/