Session Information
Session Type: Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Two separate genome-wide association studies demonstrated that polymorphisms in SERPINA1, encoding serine protease inhibitor alpha-1 antitrypsin (A1AT), are associated with increased risk of developing antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV). It has been proposed that the functionality of A1AT is decreased in inflammatory conditions. This study aimed to gain insight into function of A1AT in AAV, including the relationships of A1AT genotype, and functional activity of A1AT, with targeted biomarkers and disease activity in AAV.
Methods: Clinical data and peripheral blood samples were examined from 250 participants with AAV in a longitudinal cohort, and 80 healthy control individuals. Among those with AAV, 170 with wild-type (WT) A1AT genotype (MM) and 80 with mutant (mut) A1AT genotype (MS, MZ, SS, SZ, or ZZ) with available samples during active disease and remission were selected. Comprehensive analyses were performed, including A1AT total and functional levels, markers of neutrophil activation, and cytokines/chemokines.
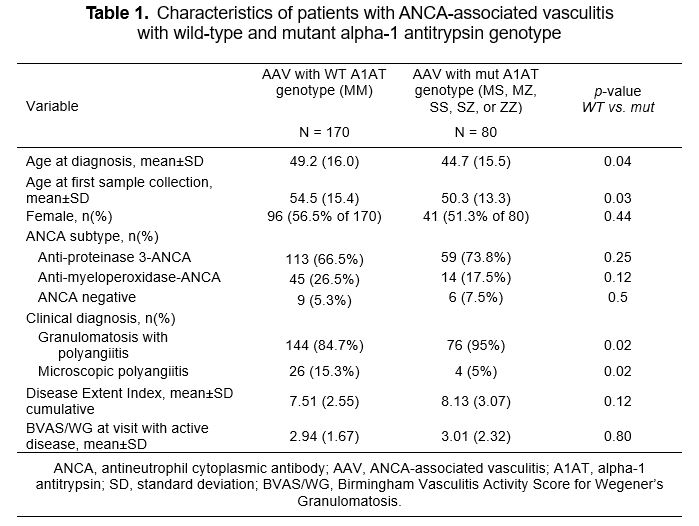
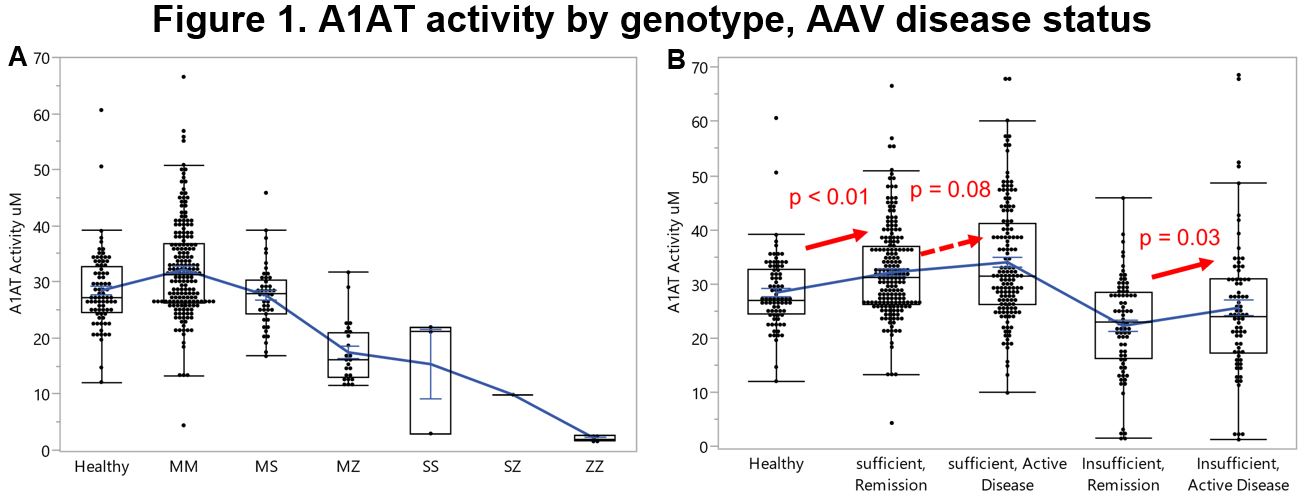
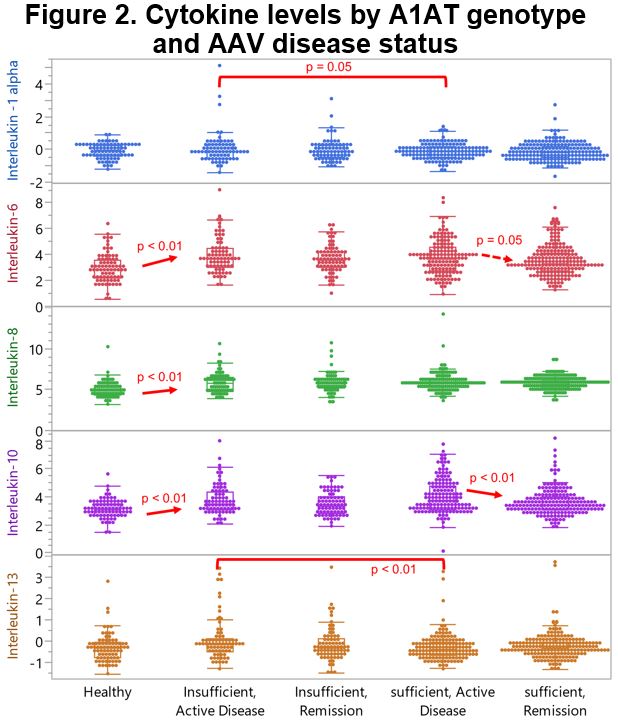
Results: Participants with mut A1AT genotype were younger at diagnosis of AAV than those with WT A1AT genotype (Table 1). Among participants with AAV and WT A1AT genotype, 66.5% were PR3-ANCA positive and 26.5% were MPO-ANCA positive during their disease course, as compared to 73.8% and 17.5%, respectively, among those with mut A1AT genotype. Birmingham Vasculitis Activity Score/WG during active visits, and Disease Extent Index are presented in Table 1. Functional A1AT was higher with WT A1AT genotype than with mut genotype during both active disease (p < 0.01) and remission (p < 0.01), and higher among participants with AAV and WT A1AT genotype than among healthy subjects (p < 0.01; Figure 1). Total and functional A1AT aligned with genotype and were consistently lower among those with Z alleles . Cytokine and chemokine levels are presented in Figure 2. During active AAV, levels of proinflammatory cytokines IL-8 and IL-6 were similar across the A1AT genotype subgroups. The decline of these cytokines during remission of AAV was more distinct among those with WT A1AT genotype than those with mut genotype. In contrast, levels of IL-1α and IL-13 were more affected among those with mut genotype than those with WT A1AT genotype, resulting in significant increased levels during active disease (p=0.05 and p < 0.01, respectively). Levels of IL-8 and IL-6 among healthy control subjects were lower than those with WT A1AT genotype during remission (p < 0.01 and p < 0.01, respectively), but IL-1α and IL-13 were similar. During active AAV and remission, levels of the anti-inflammatory cytokine IL-10 were slightly higher among those with WT A1AT genotype than those with mut genotype (p=0.07 and p=0.20, respectively). By comparison, mean IL-10 among healthy subjects was lower than among those with WT A1AT genotype in remission (p < 0.01).
Conclusion: A1AT genotype among people with AAV is associated with differences in age at diagnosis and clinical diagnosis. A1AT antigen levels and activity reflect A1AT genotypes and act as acute-phase reactants, but are not associated with disease status.
To cite this abstract in AMA style:
Fussner L, Bilic I, McAlear C, Cuthbertson D, Cheng J, Chen E, Weiller M, Specks U, Merkel P. Characterization of Alpha-1 Antitrypsin Function in ANCA-Associated Vasculitis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/characterization-of-alpha-1-antitrypsin-function-in-anca-associated-vasculitis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/characterization-of-alpha-1-antitrypsin-function-in-anca-associated-vasculitis/