Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Based on animal models, complement activation is part of Antiphospholipid Syndrome (APS) pathogenesis. However, studies investigating complement activation in antiphospholipid antibody (aPL)-positive patients are limited. Our objective was to analyze complement activation in subgroups of aPL-positive patients, using complement 3/4 (C3, C4) andcell-bound complement activation products (CB-CAPs)(B-lymphocytes [BC4d], erythrocytes [EC4d], and platelets [PC4d]).
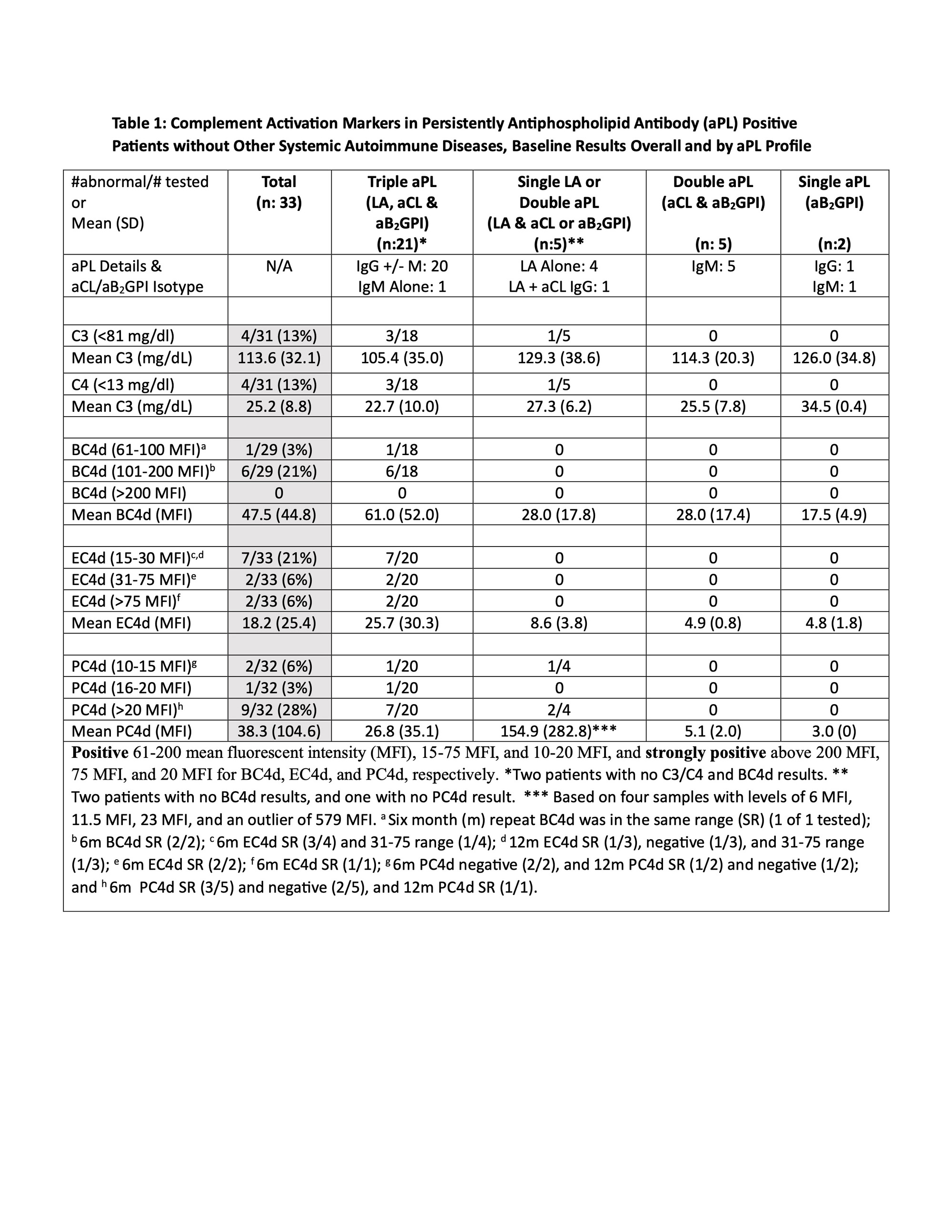
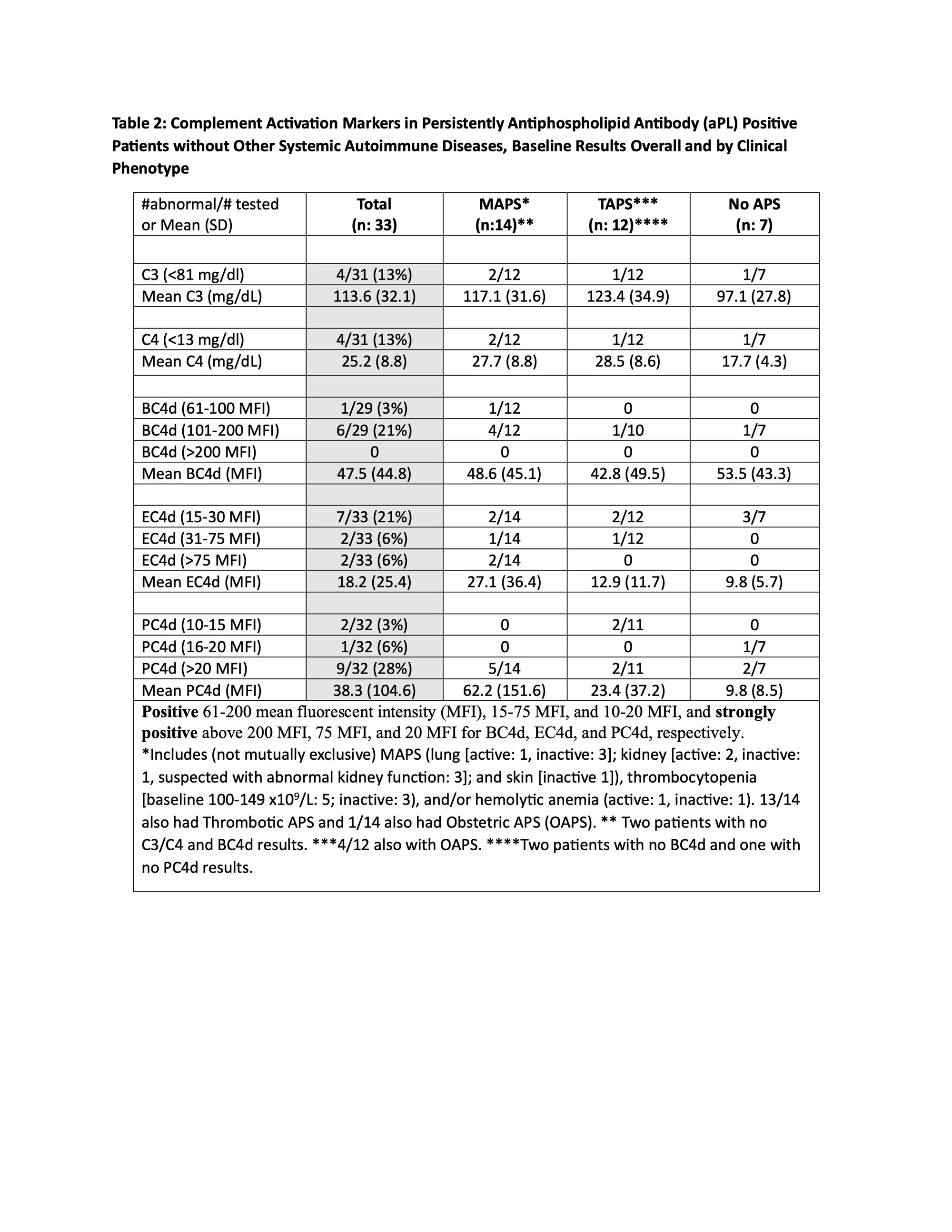
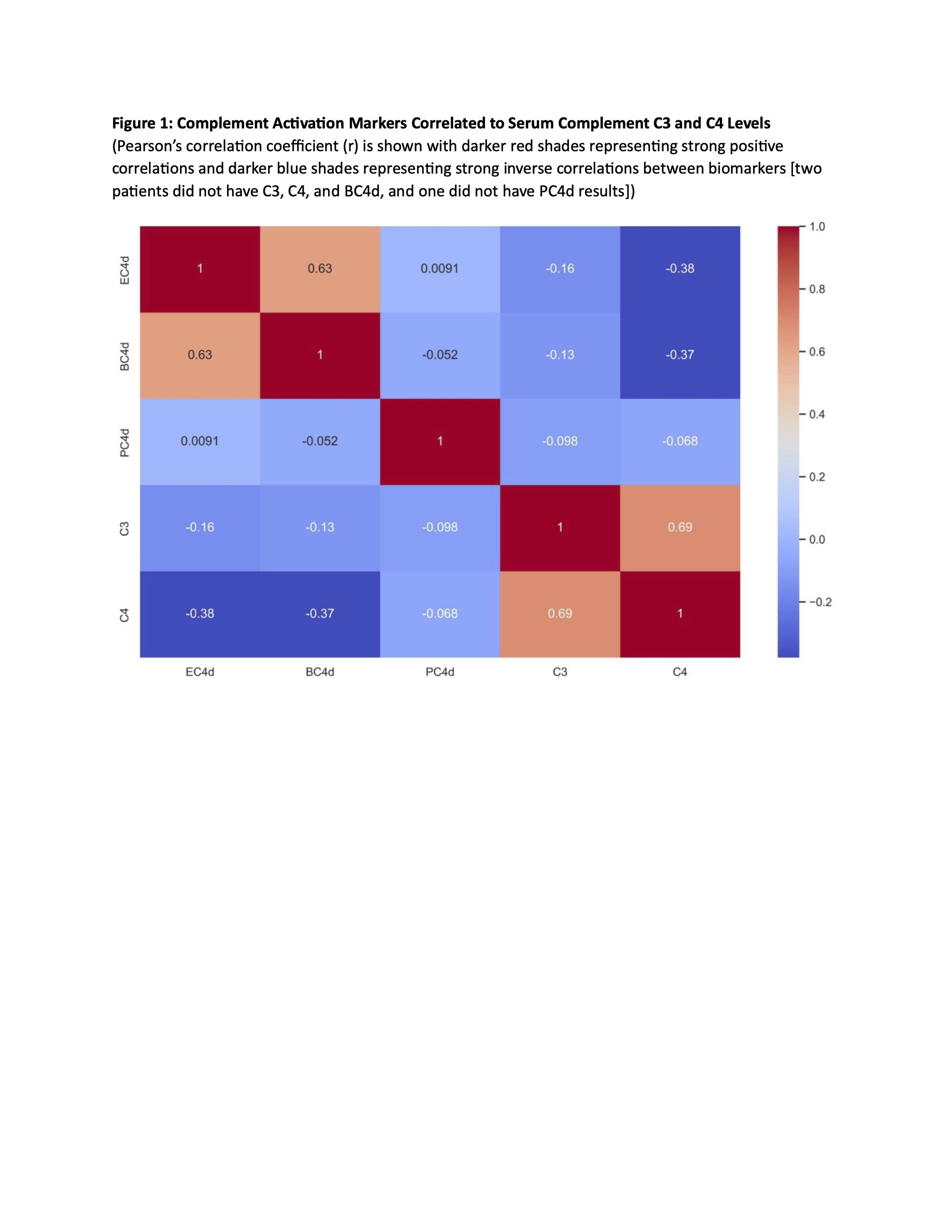
Methods: Persistently aPL-positive (>12 weeks apart; last within six months (m) prior to entry) adult patients without other systemic autoimmune diseases (SAID) were enrolled in a single center study. For those with aPL-related manifestations, i.e., microvascular APS (MAPS), thrombotic APS (TAPS), obstetric APS (OAPS), thrombocytopenia(TP) (< 150x109/L), and/or hemolytic anemia (HA),at least one event within five years prior to enrollment was required. Selected exclusion criteria wereactive infection, cancer, and corticosteroids >20mg/d. Blood and clinical data were collected at baseline; a subgroup of patients completed 6-12mfollow-up(Table 1 footnote). Positive aPLwas defined as positive lupus anticoagulant (LA) test, anticardiolipin antibody (aCL) IgG/M ≥ 40 ELISA units, and/or aβ2GPI IgG/M ≥ 40 ELISA units. C3/C4 were measured by turbidimetry and CB-CAPs by quantitative flow cytometry. Following a descriptive analysis of baseline results based on aPL profiles and clinical phenotypes, C3/C4 and CB-CAPswere correlated (Pearson).
Results: Between 8/20 and 11/22, 33 patients(female 23 [70%], mean age 50.6+12.5, White 30 [91%]) were enrolled. Four of 31 (13%) had decreased C3/C4, while 7/29 (24%)had elevated BC4d (no strong positivity [SP]), 13/33 (33%) EC4d (2% SP), and 12/32 (37%) PC4d (28% SP). Based on different aPL profiles, all patients with decreased C3/C4 or elevated BC4d, EC4d, and PC4d had triple aPL positivity, or LA positivity with/without aCL or aβ2GPI(additionally, higher mean EC4d and PC4d levels were observed in these groups) (Table 1).Based on different aPL clinical phenotypes, the number of patients with strongly positive EC4d and PC4dwere proportionally higher (14% and 36%) in those with MAPS/TP/HA, compared to those with TAPS (0 and 18%) or no APS (0 and 29%) (additionally higher mean EC4d and PC4d levels were observed in the former group) (Table 2).There was a weak correlation between C3/C4 and CB-CAPs, especially for PC4d (r=-0.098 and 0.068 for C3 and C4, respectively) (Figure 1).
Conclusion: Assessment of complement activation in persistently aPL-positive patients without other SAID demonstrated that13%, 24%, 33%, and 37% had abnormal baseline C3/C4, BC4d, EC4d, and PC4d, respectively; all in patients with triple aPL-positivity, or LA-positivity with/without aCL or aβ2GPI. Number of patients with strongly positive EC4d and PC4d were proportionally higher (14% and 36%) in those with MAPS, thrombocytopenia, and/or hemolytic anemia. Given the higher percentage of aPL-positive patients with abnormal CB-CAPs vs C3/C4, and the poor correlation between CB-CAPs and C3/C4, our study generates the hypothesis that CB-CAPs have a role in measuring disease activity in aPL-positive patients.
To cite this abstract in AMA style:
Erkan D, Vega J, O'Malley T, Concoff A. Cell-bound Complement Activation Products in Antiphospholipid Antibody-positive Patients Without Other Systemic Autoimmune Diseases [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/cell-bound-complement-activation-products-in-antiphospholipid-antibody-positive-patients-without-other-systemic-autoimmune-diseases/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cell-bound-complement-activation-products-in-antiphospholipid-antibody-positive-patients-without-other-systemic-autoimmune-diseases/