Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic Lupus Erythematosus (SLE) is characterized by the generation of autoantibodies that promote tissue injury. The development of pathogenic autoantibody-secreting B cells in lupus requires T follicular helper (Tfh) cells. Tfh cells are expanded in lupus patients with active disease and produce the cytokines IL-21 and IFN-γ that drive B cell differentiation into double negative memory (DN2) B cells, which become autoantibody-secreting cells. The regulation of pathogenic cytokine production in Tfh cells and a way to specifically target the subset producing IL-21 and IFN-γ is unknown. CD9 is a tetraspanin, shown to accumulate on activated CD4+ T cells at the immunological synapse, playing a role in their cytokine production. We identified a novel CD9-expressing Tfh cell population and assessed their role in lupus pathogenesis.
Methods: The frequency of CD9+ Tfh cells and their IL-21 and IFN-γ production was measured by flow cytometry from B6.Sle1.Yaa lupus mice at 2, 4, and 6 months of age. For assessing CD9+ circulating Tfh (cTfh) cells and DN2 B cells from patients with SLE, PBMCs were collected from patients on low doses of prednisone and hydroxychloroquine and excluding participants on immunomodulatory agents. DN2 B cell and cTfh cell phenotypes were assessed by flow cytometry or functionally by co-culturing experiments.
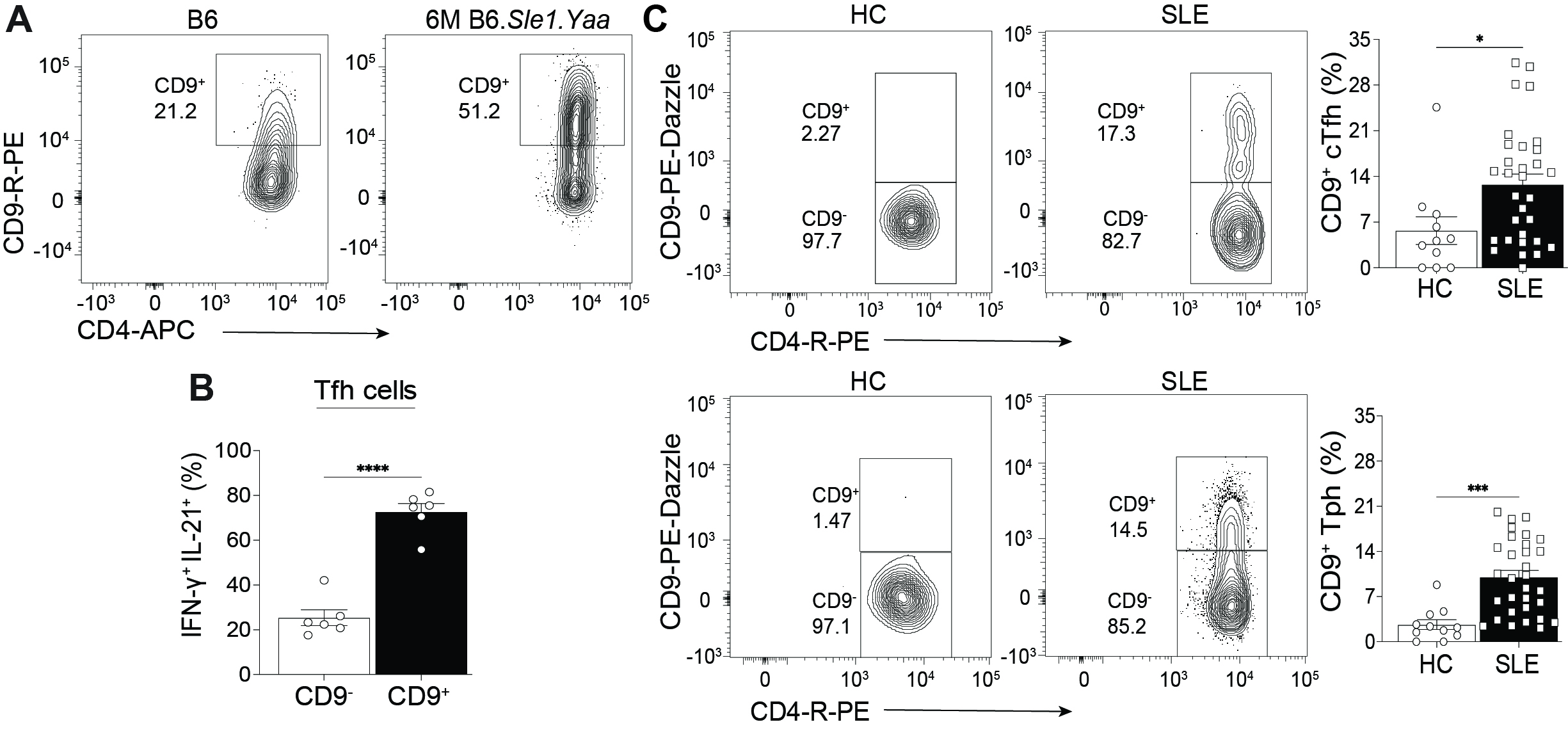
Results: We identified a distinct CD9-expressing Tfh cell (CD9+) population in murine lupus and found that they were the primary population of IL-21 and IFN-γ secreting-Tfh cells (Fig 1A, B). Comparison of CD9– and CD9+ Tfh cells revealed that CD9+ Tfh cells were more migratory to the chemokines CXCL12 and CXCL13 and expressed elevated levels of the integrin VLA-4. RNA and ATAC sequencing showed that compared to the CD9– population, CD9+ Tfh cells are a transcriptionally and epigenetically distinct population with increased cellular activation and effector Tfh cell function. Furthermore, the deletion of CD9 did not alter Tfh cell development but led to reduced IL-21 and IFN-γ production from Tfh cells and a specific decrease in the frequency of pathogenic DN2 B cells. We next assessed CD9+Tfh cells from the PBMCs of SLE patients and found an expansion of CD9+ cTfh cells and CD9+ T peripheral helper (Tph) cells compared to healthy controls (Fig 1C). The increased frequency of CD9+ cTfh cells in lupus patients was positively correlated to the increased DN2 B cell population. Moreover, co-culturing CD9+ or CD9– cTfh cells with DN2 B cells from lupus patients demonstrated that these B cells required the CD9+ cTfh and not CD9– cTfh cells for survival.
Conclusion: CD9 on Tfh cells identifies the pathogenic cytokine-producing population and regulates Tfh cell cytokine production. Additionally, CD9+ Tfh cells are an enhanced effector Tfh cell subset compared to the CD9– population. CD9+ Tfh cells remain elevated throughout the disease in murine lupus and are increased in the circulation of lupus patients, enhancing the survival of DN2 B cells. This data supports the conclusion that the CD9+ Tfh cell subset may be critical for driving disease and provides the foundation for generating novel targeted therapeutic interventions and diagnostic techniques targeting pathogenic Tfh cells.
To cite this abstract in AMA style:
Brimmer K, Antao O, Mayer D, Sanchez G, Francis R, Kyi H, Salim M, Xia B, Capitle E, Weinstein J. CD9 Expressing T Follicular Helper Cells Are a Highly Functional Subset Expanded in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/cd9-expressing-t-follicular-helper-cells-are-a-highly-functional-subset-expanded-in-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cd9-expressing-t-follicular-helper-cells-are-a-highly-functional-subset-expanded-in-systemic-lupus-erythematosus/