Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: We have described in 2013, homozygous CD59-deficient children that manifest with recurrent peripheral neuropathy resembling Guillain-Barré syndrome (GBS), with hemolytic anemia and recurrent strokes. We present the largest cohort of a 12 years of clinical follow up and basic science research in this autoinflammatory disease.
Methods: A 12-year clinical follow-up was performed in 15 patients with clinical and monthly laboratory follow up. Additional 14 patients were identified in the literature and clinical and laboratory summary was done for 29 patients. We also examined the expression of membrane-bound complement regulators in the peripheral nerves of healthy humans and a CD59-deficient patient. In addition performed single cell analysis. Single Cell RNA Sequencing was done using fresh PBMCs from a patient with CD59 homozygous mutation and an age- and gender-matched control as well as PBMCs data base. RNA from the barcoded cells from each sample was subsequently reverse-transcribed and sequencing libraries were constructed.SSingle-cell RNA-seq analyses were performed used Seurat 4.3.0. Uniform Manifold Approximation and Projection (UMAP) was then performed. Clusters were determined using the shared nearest neighbor (SNN) modularity optimization-based clustering algorithm. Differentially expressed markers were identified using the FindAllMarkers function with min.pct = 0.1, min.diff.pct = 0.1, logfc. threshold = 0.25.
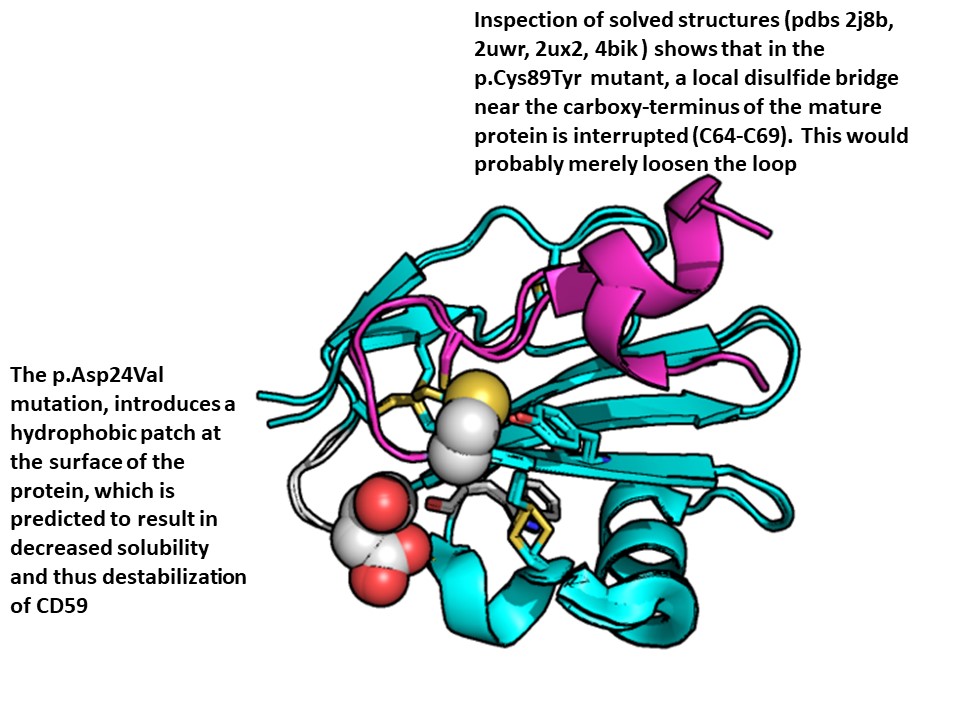
Results: Seven mutations leading to non-functional CD59 were described thus far (Figure). Patients were treated with eculizumab since 2013, ravulizumab in the last 2 years and pozelimab in the last 3 years. During 12 years of follow-up no GBS attacks and strokes, use of prednisone, and hospitalizations, were documented. Immuno-histological pathology revealed that myelin in the human peripheral nervous system is protected only by CD59 and CD55, and not by CD46 or CD35, rendering myelin in the vulnerable to complement attack and demyelination in autoinflammatory Guillain-Barre syndrome, as seen in congenital CD59 deficiency. PBMCs from the patient with homozygous CD59 deficiency showed marked depletion of NK cells (36% of normal) and marked elevation in CD4:CD8 ratio; 6.14 compared to 3.75 in normal control. Tregs were twice in number in the patient and both CD4 and CD8 cells expressed differentially gene clusters. B cells were a little bit elevated in the patient, but naïve B cells were elevated 2.5 folds. In one cluster SNHG5, RPS28, ARL4C, HLA-DPB1, AES, ABRACL, CSTB, BAX, CAP1, were elevated along with others that were differentially negatively expressed. Monocytes were relatively depleted in the patient. These gene changes fitted to known patterns in antigen presentation, TH17 signaling pathway, type I diabetes, and other autoimmunity, GvHD, and some viral and bacterial infections.
Conclusion: Patients with congenital CD59 deficiency should be treated by anti-C5 monoclonal antibodies, otherwise they will die in few years following initial symptoms. SCA reveals expected and unexpected changes in PBMC subpopulations from membrane CD59 deficient patient. These finding may elucidate unknown functions of both canonic and non-canonic CD59 functions.
To cite this abstract in AMA style:
Mevorach D, Karbian N, Zeibak M, Tabib A. CD59 Complement Membrane Regulator Deficiency: 12 Years of Clinical and Molecular Follow-up of 29 Patients [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/cd59-complement-membrane-regulator-deficiency-12-years-of-clinical-and-molecular-follow-up-of-29-patients/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cd59-complement-membrane-regulator-deficiency-12-years-of-clinical-and-molecular-follow-up-of-29-patients/