Session Information
Session Type: Abstract Session
Session Time: 12:45PM-1:00PM
Background/Purpose: Inflammatory skin diseases vary widely in symptoms and causes. While ultraviolet (UV) light helps treat some like vitiligo and psoriasis, in conditions like cutaneous lupus erythematosus (CLE) and dermatomyositis (DM), UV exposure can worsen disease. The biological basis for this photosensitivity remains unclear.
Methods: We used a multi-omics approach—single-cell RNA sequencing (scRNA-seq), spatial transcriptomics, and proteomics—to analyze lesional and non-lesional skin from patients with DM, CLE, vitiligo, psoriasis, and healthy controls. In vivo, a photoprovocation test was performed on sun-protected skin of healthy volunteers, DM, and CLE patients; post-irradiation biopsies were analyzed by flow cytometry and spatial transcriptomics. In vitro, keratinocytes were treated with UVB ± IFN-β. Supernatants were applied to monocyte-derived dendritic cells (moDCs) generated from PBMCs, and their activation was assessed.
Results: CD14⁺ myeloid cells, which resembled monocyte-derived dendritic cells (moDCs) in culture, were the only immune population distinguishing photosensitive (CLE, DM) from photoresponsive (psoriasis, vitiligo) diseases. These cells were enriched in lesional DM (43%) and CLE (12%) compared to controls ( < 0.3%) and localized near the dermo-epidermal junction, adjacent to cytotoxic CD4⁺ T cells. CD14⁺ myeloid cells expressed IFNB1 and MMP9, suggesting involvement in IFN-I signaling and tissue remodeling. UVB photoprovocation confirmed increased recruitment of CD14⁺ myeloid cells to the skin, more so in DM and CLE patients. IFN-β priming sensitized keratinocytes to UVB-induced death and enhanced secretion of TNF-α and IL-6, which activated moDCs. Upon activation, moDCs release chemokines promoting immune cell recruitment, amplified in the presence of IFN-β.
Conclusion: Our findings reveal a UVB-induced inflammatory circuit driven by IFN-β–primed keratinocytes and CD14⁺ myeloid cells in CLE and DM. By defining this keratinocyte–myeloid interaction, we highlight new opportunities for targeted therapies that may prevent lesion flares in photosensitive patients.
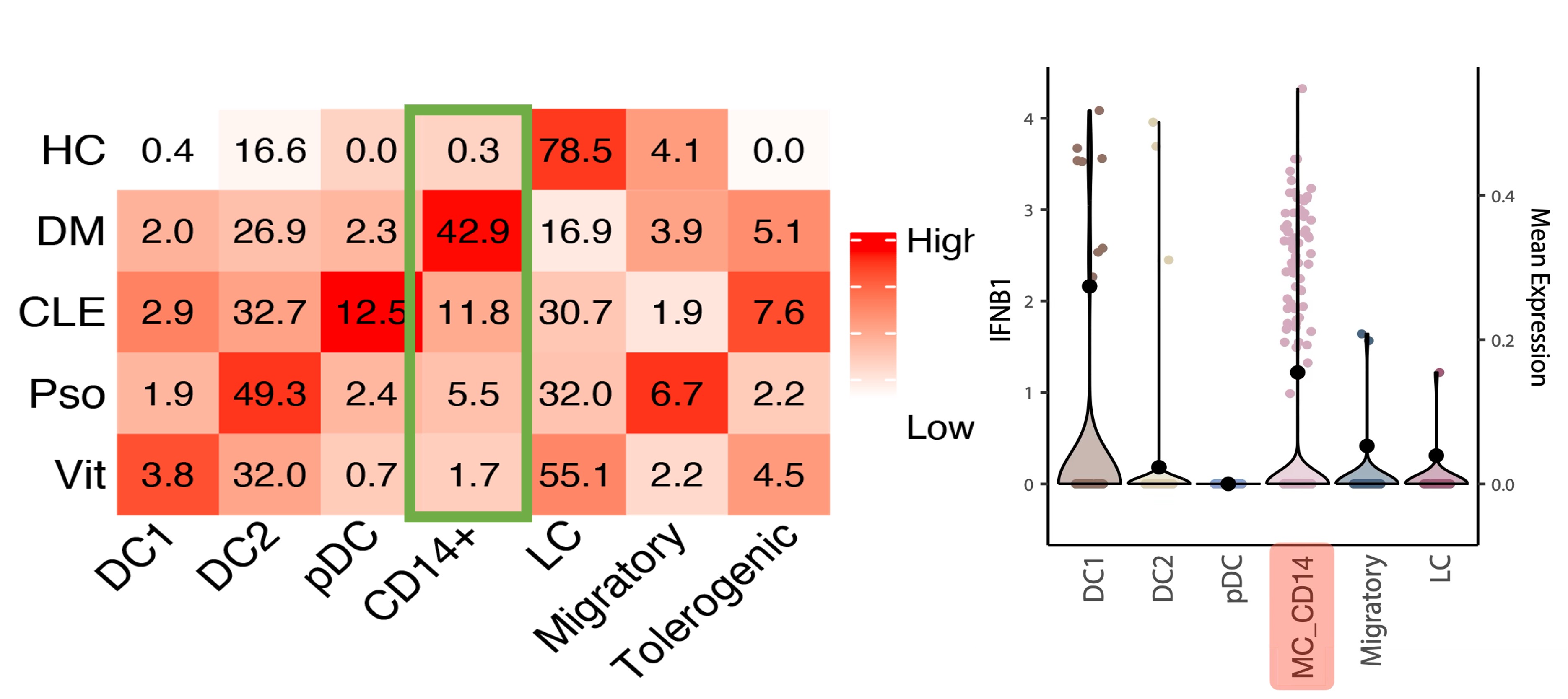 CD14⁺ myeloid cells (MC_CD14) are expanded in dermatomyositis and lupus skin in our single-cell RNA sequencing dataset (heatmap, left) and show the highest IFNB1 expression among dendritic cell subsets (violin plot, right).
CD14⁺ myeloid cells (MC_CD14) are expanded in dermatomyositis and lupus skin in our single-cell RNA sequencing dataset (heatmap, left) and show the highest IFNB1 expression among dendritic cell subsets (violin plot, right).
.jpg) The dot plot (top) shows shared marker expression between CD14⁺ myeloid subsets identified by scRNA-seq and spatial single-cell transcriptomics (SeqFISH+). The MC_Inf transcriptional signature aligns with the scRNA-seq-defined CD14⁺ myeloid profile and maps to cells spatially enriched near the dermo-epidermal junction, as illustrated in the bottom panels.
The dot plot (top) shows shared marker expression between CD14⁺ myeloid subsets identified by scRNA-seq and spatial single-cell transcriptomics (SeqFISH+). The MC_Inf transcriptional signature aligns with the scRNA-seq-defined CD14⁺ myeloid profile and maps to cells spatially enriched near the dermo-epidermal junction, as illustrated in the bottom panels.
.jpg) Schematic and heatmaps showing moDCs stimulated with supernatants from UV-treated keratinocytes. Exposure to supernatants from UVB-damaged keratinocytes, particularly in the presence of IFN-β, induced a transcriptional program enriched for MC_Inf markers (e.g., CD80, CD86, CCL4, MMP9), consistent with acquisition of a pro-inflammatory phenotype.
Schematic and heatmaps showing moDCs stimulated with supernatants from UV-treated keratinocytes. Exposure to supernatants from UVB-damaged keratinocytes, particularly in the presence of IFN-β, induced a transcriptional program enriched for MC_Inf markers (e.g., CD80, CD86, CCL4, MMP9), consistent with acquisition of a pro-inflammatory phenotype.
To cite this abstract in AMA style:
Haddadi N, Afshari K, Wang Y, Lopes C, Eng C, Martinez-Gutierrez N, Whiteman L, Wei K, Frieda K, Gallucci S, Rosenbach M, Vleugels R, Harris J, Garber M, Rashighi M. CD14⁺ Myeloid Cells Mediate UVB Photosensitivity in Autoimmune Skin Disease via a Spatially Resolved Inflammatory Circuit [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/cd14%e2%81%ba-myeloid-cells-mediate-uvb-photosensitivity-in-autoimmune-skin-disease-via-a-spatially-resolved-inflammatory-circuit/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cd14%e2%81%ba-myeloid-cells-mediate-uvb-photosensitivity-in-autoimmune-skin-disease-via-a-spatially-resolved-inflammatory-circuit/
