Session Information
Date: Tuesday, November 9, 2021
Title: Osteoarthritis & Joint Biology – Basic Science Poster (1468–1479)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Changes in subchondral bone structure (SCB) occur in osteoarthritis (OA), and are visible by imaging as subchondral sclerosis on radiographs, and bone marrow lesions (BMLs) by MRI. These changes are the result of active bone remodeling that occurs in OA. BMLs are correlated with pain in OA patients. We previously demonstrated that bone remodeling was significantly reduced after joint injury in mice deficient in CD14. CD14 enhances Toll-like receptor (TLR) mediated inflammation by acting as a co-receptor for multiple TLRs, increasing TLR sensitivity to damage associated molecular patterns (DAMPS) to activate proinflammatory signaling pathways. Soluble CD14 (sCD14), TLRs, and DAMPs (TLR ligands) are all elevated in OA, and sCD14 has been implicated as a biomarker of symptom and structural severity in OA. Increasing evidence suggests TLR signaling influences bone remodeling as well, through activation of osteoclast precursor cells, but the specific role of CD14 on this process is unknown. Here, we investigate the potential mechanism by which CD14 influences bone remodeling through in vitro analysis of osteoclastogenic and osteoblastic potential.
Methods: Bone marrow (BM) hematopoietic and mesenchymal precursors were isolated from long bones of male wild-type C57BL/6 (WT) and CD14-deficient (CD14-/-) mice (12 weeks). BM cells flushed from long bones were cultured for 1 day to separate non-adherent from adherent population. Osteoclast differentiation (non-adherent cells) was stimulated by M-CSF and RANKL for 5 days. Osteoblastic cultures (adherent cells) were stimulated with β-glycerophosphate, ascorbic acid, and Dexamethasone for 19 days. Osteoclasts were identified as TRAP-positive multinucleated cells. Osteoclast activity was assessed by resorption assays on bone slices and toluidine blue staining. Osteoblast cultures were stained with Alizarin red to detect mineralization. RT-qPCR analysis was performed to detect mRNA levels of osteoclast (calcitonin receptor/CTR and cathepsin K/Ctsk) and osteoblast (osteopontin/OPN and osteocalcin/BLAP) differentiation markers.
Results: CD14-/- BM cells had reduced number of TRAP positive and multinucleated cells (p = 0.01; Fig. 1 A-D) and reduced Ctsk mRNA (p = 0.03; Fig. 1 E&F), compared to WT. CD14-/- cells showed a 50% reduction in resorption activity compared to WT (Fig. 1 G-I). No significant differences were observed between strains in osteoblastic potential measured by mineralization and mRNA levels of osteoblastic markers (Fig. 2).
Conclusion: CD14-/- BM precursor cells have reduced capacity to differentiate into osteoclasts, while osteoblastic potential is retained. This suggests that the reduced capacity of CD14-/- osteoclastic cells may impact bone remodeling in response to joint injury by disrupting the balance between resorption and formation. Future experiments will determine the influence of DAMPs relevant to OA pathogenesis on osteoclastogenesis, and the density of osteoclasts and precursors in SCB after injury. These experiments will lead to a better understanding CD14/ TLR signaling in SCB remodeling following joint injury and OA, and the potential for CD14 blockade to prevent pathologic bone remodeling in disease.
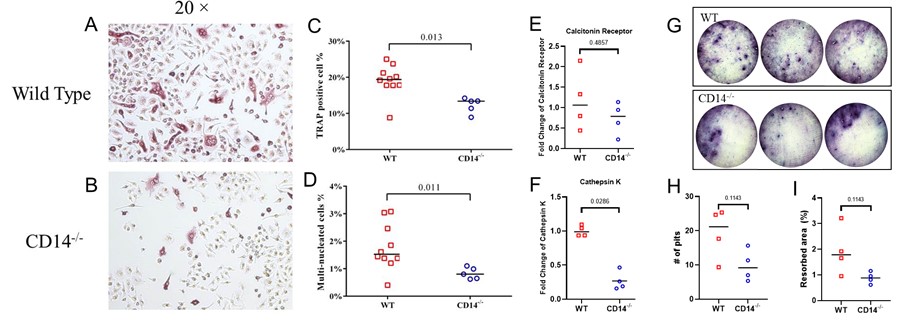 Representative TRAP staining from (A) wild type mice and (B) CD14-/- mice. (C) TRAP positive cells and (D) multinucleated cells quantitated by automated image analysis using ImageJ software. mRNA for CTR (E) and Ctsk (F) quantitated by qPCR. (G) Osteoclast activity was assayed by quantifying the (H) number of resorption pits and (I) resorption area on bone slices stained with toluidine blue.
Representative TRAP staining from (A) wild type mice and (B) CD14-/- mice. (C) TRAP positive cells and (D) multinucleated cells quantitated by automated image analysis using ImageJ software. mRNA for CTR (E) and Ctsk (F) quantitated by qPCR. (G) Osteoclast activity was assayed by quantifying the (H) number of resorption pits and (I) resorption area on bone slices stained with toluidine blue.
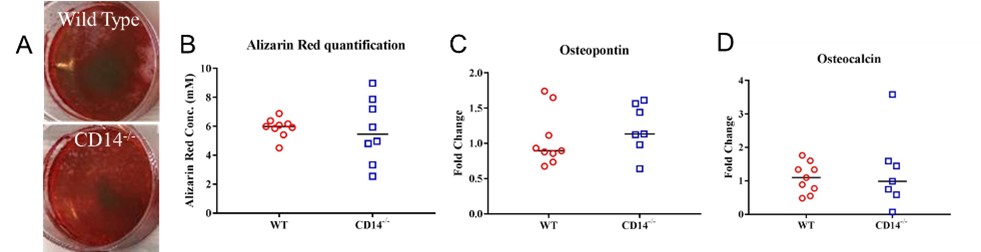 (A&B) Alizarin Red staining of WT and CD14-/- BMMSCs after differentiation. No differences were observed between strains. This was confirmed by measuring mRNA levels of osteoblastic markers, (C) osteopontin and (D) osteocalcin.
(A&B) Alizarin Red staining of WT and CD14-/- BMMSCs after differentiation. No differences were observed between strains. This was confirmed by measuring mRNA levels of osteoblastic markers, (C) osteopontin and (D) osteocalcin.
To cite this abstract in AMA style:
Martinez J, Nguyen V, Zhou C, Dodge G, Scanzello C. CD14, a Toll-like Receptor Co-Factor, Influences Osteoclast Differentiation and Activity, but Does Not Alter Osteoblastic Potential of Bone-Marrow Precursors in Mice [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/cd14-a-toll-like-receptor-co-factor-influences-osteoclast-differentiation-and-activity-but-does-not-alter-osteoblastic-potential-of-bone-marrow-precursors-in-mice/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cd14-a-toll-like-receptor-co-factor-influences-osteoclast-differentiation-and-activity-but-does-not-alter-osteoblastic-potential-of-bone-marrow-precursors-in-mice/
