Session Information
Date: Saturday, November 12, 2022
Title: Abstracts: SLE – Diagnosis, Manifestations, and Outcomes I: Renal Aspects
Session Type: Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Medication management strategies in patients with lupus-related end stage kidney disease (ESKD) have not been studied. Our prior work has shown that HCQ may be underutilized and corticosteroids may be overutilized in these patients. We aimed to assess whether patients with lupus nephritis who continue HCQ after ESKD have lower risk of cardiac outcomes, thrombosis, infections, and overall mortality compared to those maintained on CS alone or those on both CS/HCQ after ESKD onset.
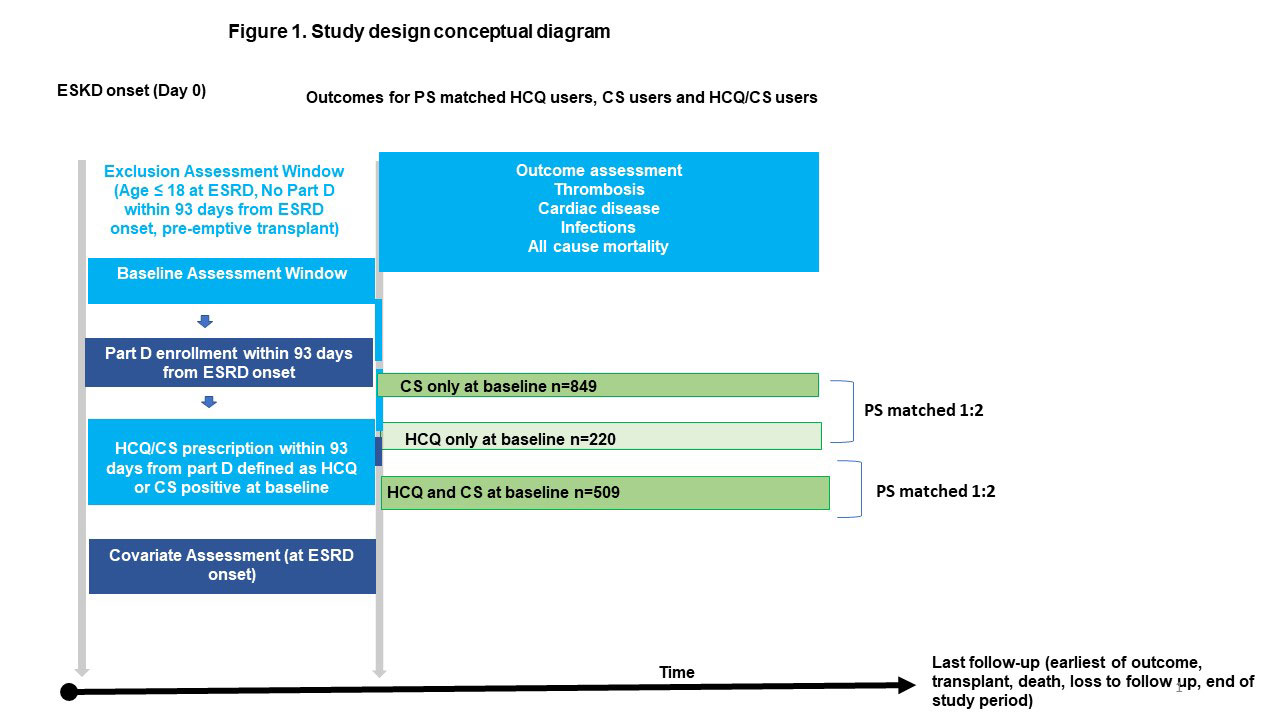
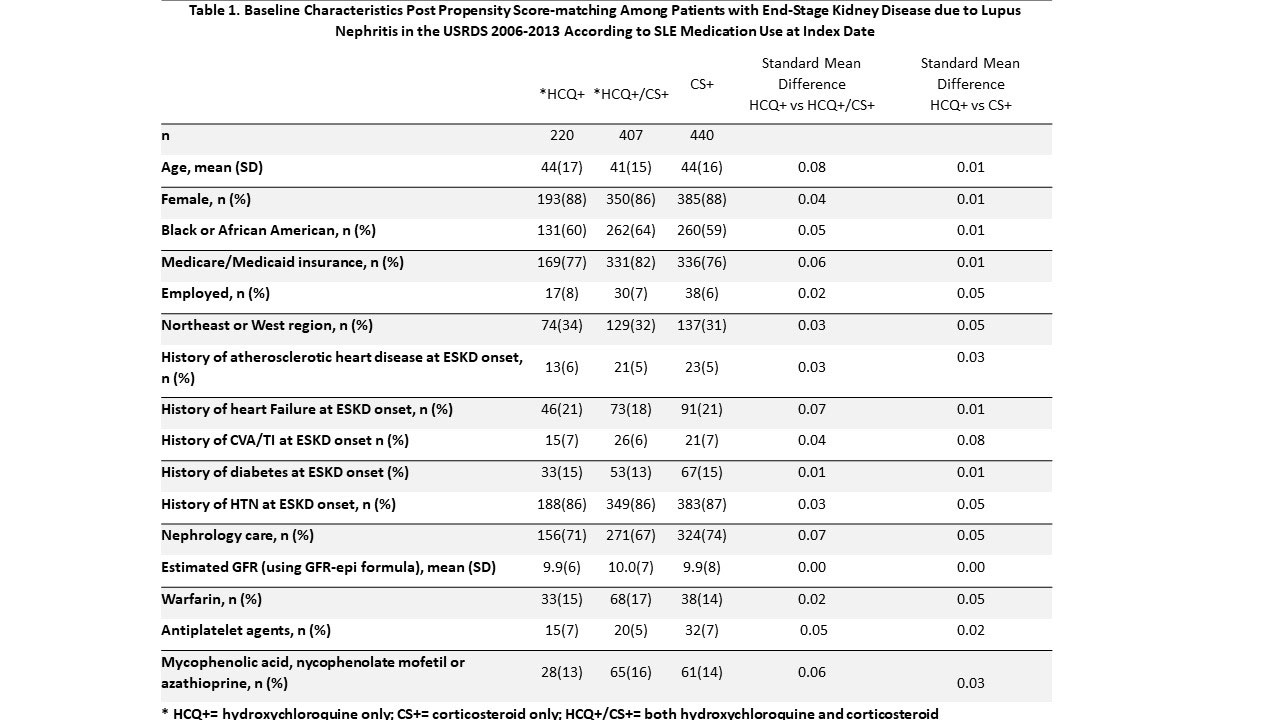
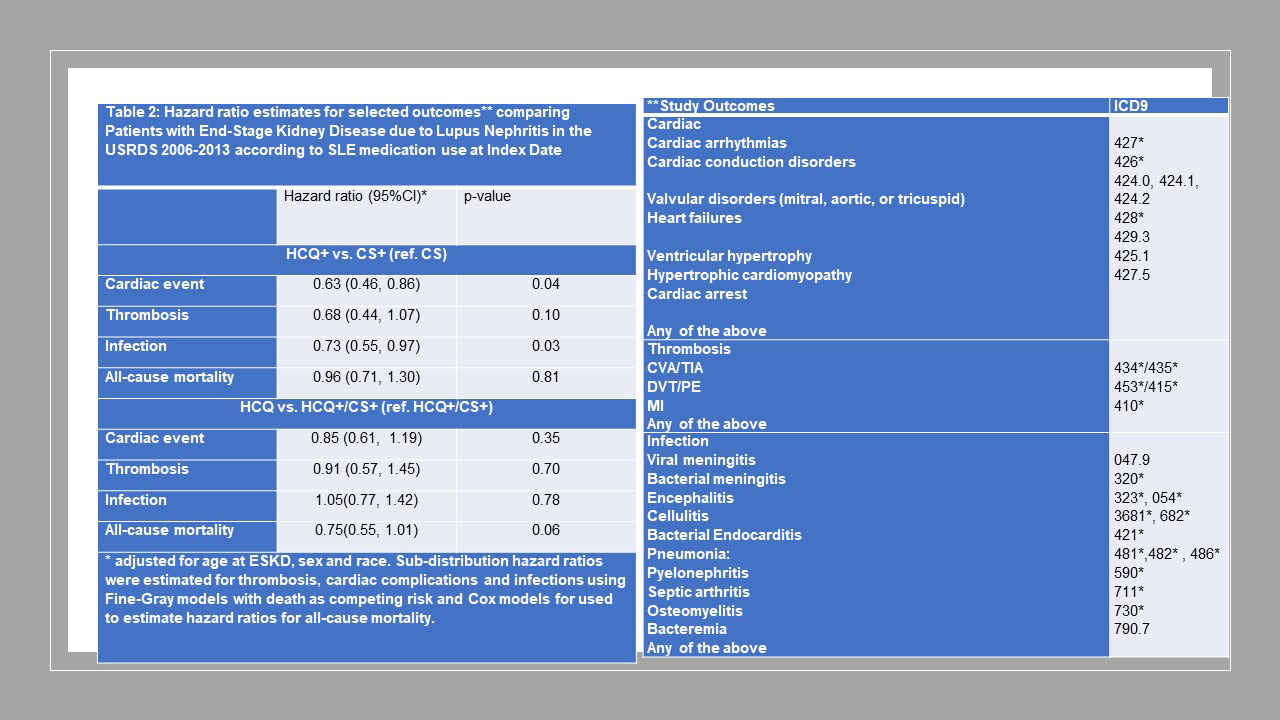
Methods: We studied SLE patients over 18 years old enrolled in the United States Renal Disease Systems (USRDS) database using data from January 2006–December 2013. Patients were defined as having hydroxychloroquine (HCQ) or corticosteroid (CS) prescription at ESKD onset (index date) if they were 1) enrolled in Medicare Part D within 93 days from ESKD onset and 2) had a HCQ or CS prescription within 93 days from the Part D start index date (Fig 1). Three comparison groups were identified based on the index date medication status: HCQ only (HCQ+), CS only (CS+), and HCQ+/CS+. HCQ+ group was propensity score-matched (PS-matched) on demographics, medications and comorbidities with the CS+ group and with the HCQ+/CS+ group. Pairwise PS analyses were performed (HCQ+ vs. CS+, HCQ+ vs HCQ+/CS+). Sub-distribution hazard ratios (HR) were estimated using Fine-Gray models for thrombosis, cardiac complications, infections separately while accounting for competing risk of death. Cox regression models were used for all-cause mortality.
Results: Of 1578 eligible patients studied, 220 were HCQ+, 849 were CS+ and 509 were HCQ+/CS+; 220 HCQ+ were PS-matched with 440 CS+; 219 HCQ+ were PS-matched with 407 HCQ+/CS+ (Table 1) Compared to the CS+ group, the HCQ+ group had a lower risk of cardiac complications and infections (Table 2). There were no differences with respect to thrombotic complications and all-cause mortality. There were no differences between HCQ+ and HCQ+/CS+ group with respect to these outcomes.
Conclusion: Patients with lupus nephritis who were maintained on CS alone after ESKD onset had an increased risk of cardiac complications and mortality compared to propensity-score matched patients maintained on HCQ alone. These results are consistent with past findings in non-ESKD patients with lupus nephritis. The results also suggest that HCQ use in ESKD was not associated with increased cardiac toxicity despite decreased renal clearance in these patients. Major limitations (inherent to USRDS) include lack of data on SLE-specific variables, such as disease duration and activity, and prevalent user design (the duration of HCQ/CS exposure prior to ESKD is not known). Nevertheless, the results may provide a rationale for minimizing CS exposure in SLE ESKD.
To cite this abstract in AMA style:
Broder A, Mowrey W, Yoshida K, Costenbader K. Cardiac Complications, Thrombosis, Infections and All-cause Mortality Among Patients with End-Stage Kidney Disease Due to Lupus Nephritis in the USRDS 2006-2013 According to SLE Medication Use [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/cardiac-complications-thrombosis-infections-and-all-cause-mortality-among-patients-with-end-stage-kidney-disease-due-to-lupus-nephritis-in-the-usrds-2006-2013-according-to-sle-medication-use/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cardiac-complications-thrombosis-infections-and-all-cause-mortality-among-patients-with-end-stage-kidney-disease-due-to-lupus-nephritis-in-the-usrds-2006-2013-according-to-sle-medication-use/