Session Information
Date: Monday, November 14, 2022
Title: Abstracts: T Cell Biology and Targets in Autoimmune and Inflammatory Disease
Session Type: Abstract Session
Session Time: 3:00PM-4:00PM
Background/Purpose: Systemic lupus erythematosus (SLE) is characterized by a dysregulated T cell compartment and production of autoantibodies leading to organ damage. We previously identified that activity of Calcium/Calmodulin-dependent protein kinase (CaMK4) is increased in SLE T cell and that a Camk4 global knockdown leads to decreased autoantibody levels and improved lupus-like disease in the MRL/lpr lupus-prone mouse (1). Since CaMK4 is not expressed in the B cell compartment, we hypothesized that CaMK4 was important for the T-dependent B cell response.
Methods: Gene expression was evaluated using quantitative-reverse transcriptase PCR. We used two T cell-dependent antigens, sheep red blood cells (SRBC) and NP-Chicken gamma globulin (NP-CGG), to immunize C57BL/6 mice with a T-cell specific Camk4 knockdown (B6.Camk4fl/fl.dlckCre) or not (B6.Camk4fl/fl) by intraperitoneal injection. Following immunization, we studied the T follicular helper (Tfh; CD4+CD44+CXCR5+PD1+) T cells and germinal center (GL7+CD95+) B cells populations in the spleen and cervical lymph nodes (cLN) using flow cytometry. Luciferase assay using the Bcl6 promoter cloned on the pGL3 vector was used to investigate the control of Bcl6 transcription .Serum IgM and IgG responses to NP-CGG were evaluated using ELISA. To translate our findings to autoimmunity, we used the lupus-like B6.lpr murine model crossed with the Camk4fl/fl.dlckCre mouse and studied the Tfh compartment, autoantibody levels and the severity of the disease.
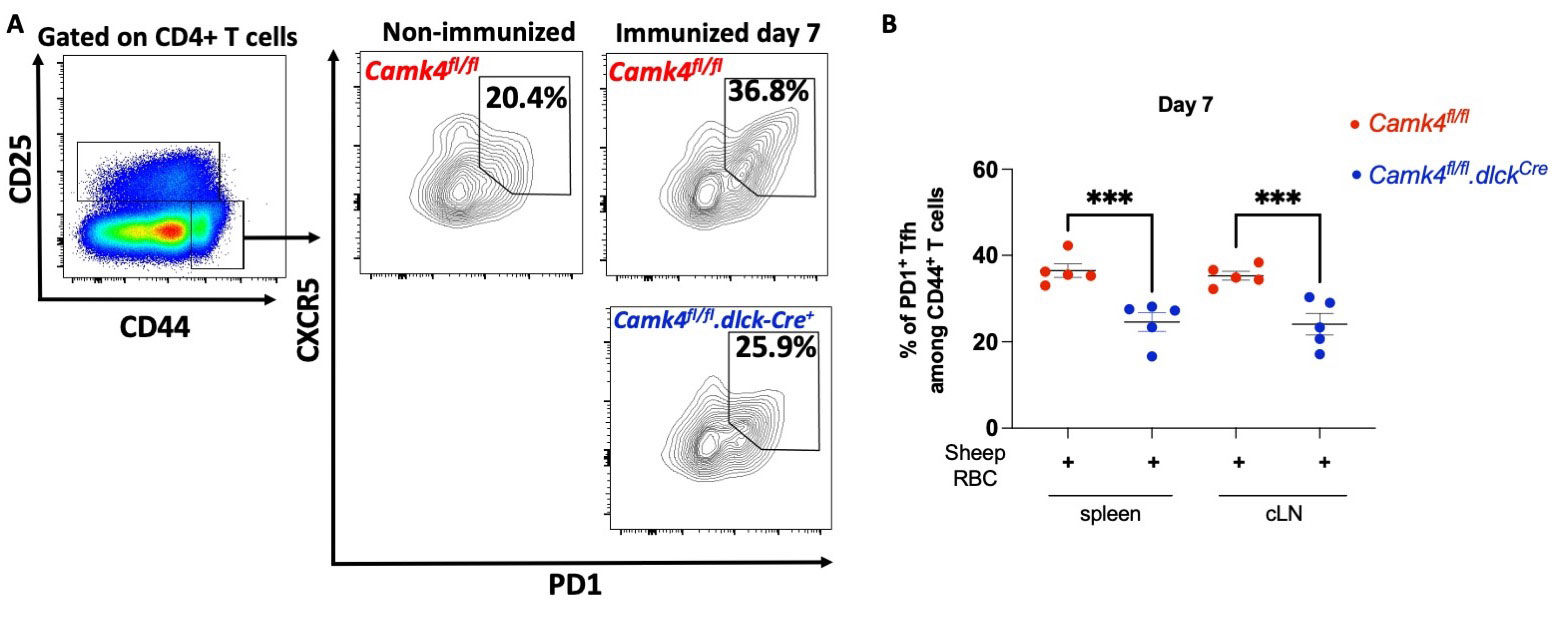
Results: We identified that primary murine Tfh express high level of Camk4, similar to Tconv and T regulatory cell, and unlike B cells which do not express any. Compared to CaMK4-sufficient littermates, B6.Camk4fl/fl.dlckCre mice were characterized by a dampened expansion of the Tfh compartment 7 days post-SRBC immunization, in the spleen and cLN (p < 0.001 for both comparisons; Fig. 1A-B). Conversely, immunized B6.Camk4fl/fl.dlckCre had decreased germinal center B cells compared to Cre-negative littermates. In terms of humoral response, B6.Camk4fl/fl.dlckCre had decreased IgG1 and IgG2b response to NP-CGG immunization. Mechanistically, preliminary data suggest that the Bcl6 gene expression is controlled by CaMK4 through the transcriptional repressor CREMa, as determined by a luciferase reporter assay. In lupus B6.lpr mice, T-cell specific knockdown of Camk4 led to decreased circulating, splenic and cLN Tfh and germinal center cells without any change in the FoxP3+ regulatory Tfh cells. Additionally, splenic plasma cells and age-related B cells were decreased in B6.lpr.Camk4fl/fl.dlckCre mice compared to CaMK4-sufficient littermates. This translated into decreased anti-dsDNA IgG1 and IgG2b levels, decreased IgG/C3 staining in kidney immunofluorescence and improved kidney pathology.
Conclusion: We identified that CaMK4 expression is instrumental for the expansion of the Tfh compartment, and for the promotion in T-dependent B cell response in the mouse models of immunization and autoimmunity. The characterization of molecular mechanisms underlying systemic autoimmunity may lead the identification of new therapeutic opportunities in SLE.
Reference:
1. Koga T, et al. J Immunol. 2012 Oct 1;189(7):3490–6.
(A) Gating strategy for the T follicular helper cell analysis. (B) Cumulative results of splenic and cLN activated Tfh 7 days after immunization. Each point represents one mice and bars indicate s.e.m. ***, p < 0.001 using one-way ANOVA with Sidak's correction.
To cite this abstract in AMA style:
Scherlinger M, Li H, Pan W, Tsokos M, Tsokos G. CaMK4 Controls the Humoral Response via the T Follicular Helper Compartment and Regulates Autoimmunity [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/camk4-controls-the-humoral-response-via-the-t-follicular-helper-compartment-and-regulates-autoimmunity/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/camk4-controls-the-humoral-response-via-the-t-follicular-helper-compartment-and-regulates-autoimmunity/