Session Information
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Osteoporotic fractures contribute to significant morbidity and mortality. Dual-energy x-ray absorptiometry (DXA) is a valuable tool to identify osteoporosis, assess fracture risk, and monitor treatment response. High-quality DXA requires attention to DXA acquisition, analysis, interpretation, and reporting, but errors at each step are common, and can adversely affect patient care. This quality improvement study evaluated national VA DXA equipment and processes of care, as part of a larger evaluation to understand access to and quality of DXA services in the VA, and for rural Veterans.
Methods: An interdisciplinary work group of radiologists, nuclear medicine physicians, social scientists, endocrinologists, and rheumatologists iteratively developed the survey identifying domains, developing items, and revising for parsimony. This quality improvement project was reviewed by the VA Office of Rural Health and University of Iowa IRB. VA nuclear medicine and radiology leadership at 178 sites were emailed invitations and given 60 days to complete the survey online.
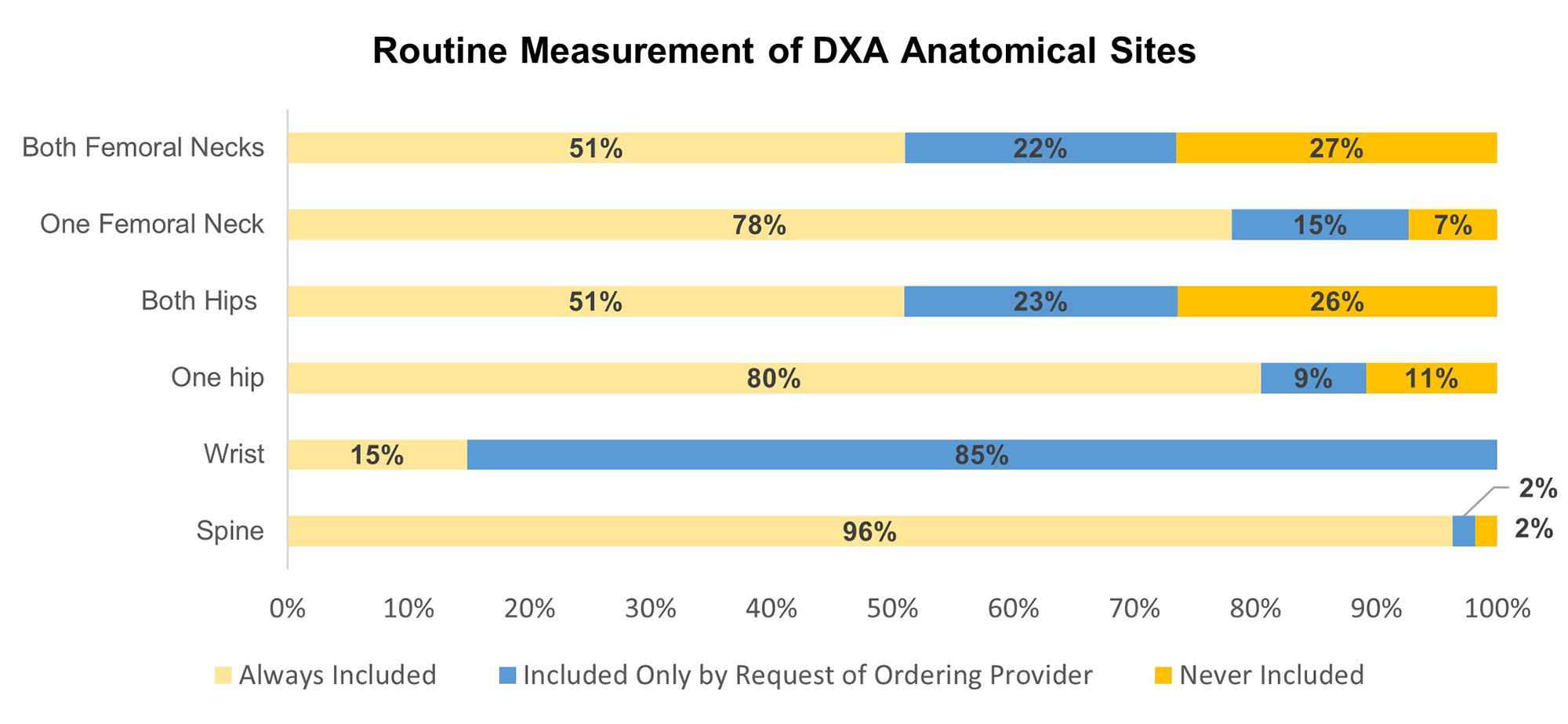
Results: Sixty-three sites (35%) completed the survey. The most common DXA manufacturers were Hologic and GE; 76% were rated as in “excellent” or “good” condition. Mean DXA scanner age was 6 years (SD 4.35). Use of a validated fracture risk measure such as FRAX® was reported by 75% of sites. Few sites had the capability for vertebral fracture assessment, trabecular bone score, or atypical femur fracture evaluation (Table 1). Lack of equipment routinely used with DXA acquisition (e.g., height/weight measurement, patient transfer, and proper positioning) was reported by 19% of facilities. Most (80%) facilities reported no equipment needs, yet few sites routinely used a stadiometer or scale at the time of DXA (Table 1). Few sites (41%) routinely send patients instructions to prepare them for their DXA appointment. For those sites, 54-58% provided information to assist with wayfinding and proper dress for image acquisition, but few provided instructions advising patients to avoid contrast, barium, and calcium supplements prior to DXA. DXA indication was the most frequent order element collected. Most routinely collect information about bone health medications used to manage osteoporosis (75%), medications affecting bone remodeling (76%), and osteoporosis risk factors (78%) at the time of DXA, but there was high variability among specific medications and risk factors (Table 2). The spine (96%), one total hip (80%), and one femoral neck (78%) were the most common routine anatomical sites for DXA (Figure 1).
Conclusion: Though many VA DXA facilities are maintaining standard of care practices regarding equipment and processes of care, much variability remains, particularly in routine use of equipment for accurate measurement, pre-visit patient education, and collection of clinical information important to the interpretation of DXA results. This study was limited by number of respondents -roughly one-third of potential VA DXA sites. Study results will be used to facilitate coordination across radiology and specialty care services to provide Veterans with high quality osteoporosis DXA screening and care processes that facilitate access.
To cite this abstract in AMA style:
Miller K, Steffen M, McCoy K, Mengeling M, Davila H, Wardyn S, Solimeo S. Bone Density Imaging: Evaluation of Equipment and Process of Care Quality in US Veterans [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/bone-density-imaging-evaluation-of-equipment-and-process-of-care-quality-in-us-veterans/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bone-density-imaging-evaluation-of-equipment-and-process-of-care-quality-in-us-veterans/