Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Toll like receptor (TLR) 7 and TLR8 are activated as part of the disease pathophysiology of systemic lupus erythematosus (SLE), including lupus nephritis (LN), and related autoimmune diseases, such as Sjögren’s Syndrome. BMS-986256, an orally administered, potent and selective inhibitor of TLR7/8, has demonstrated efficacy in animal models of lupus as well as favorable PK and acceptable safety profiles in healthy subjects. It is now in clinical development for the treatment of SLE. Mycophenolate mofetil (MMF) is an immunosuppressive agent that is widely used in immune‑mediated diseases and is often used in the treatment of patients with more serious presentations of active SLE. MMF gets converted to its active metabolite mycophenolic acid (MPA) following oral administration. Therapeutic drug monitoring for MMF and MPA is recommended in certain cases, especially when used as a first-line therapy. Given that MMF is often used in the treatment of SLE, it is likely that BMS-986256 will be administered concomitantly with MMF in Phase 2 studies, and more importantly, in clinical practice. Thus, the effect of BMS-986256 on the exposures of the MMF active metabolite MPA and the phenolic glucuronide of MPA (MPAG) were evaluated.
Methods: This was an open label, single sequence crossover study in which 24 healthy participants received a single oral dose of MMF 1000 mg on Day 1. Starting on Day 5 through Day 28, participants received 30 mg BMS-986256 QD oral dose. On Day 26, subjects received a second oral dose of MMF 1000 mg (in addition to QD dose of BMS-986256). Blood samples were collected after each treatment for PK evaluation. Safety evaluations (adverse events (AEs)), physical and skin examinations, vital signs, electrocardiograms, laboratory tests) were performed during the course of the study.
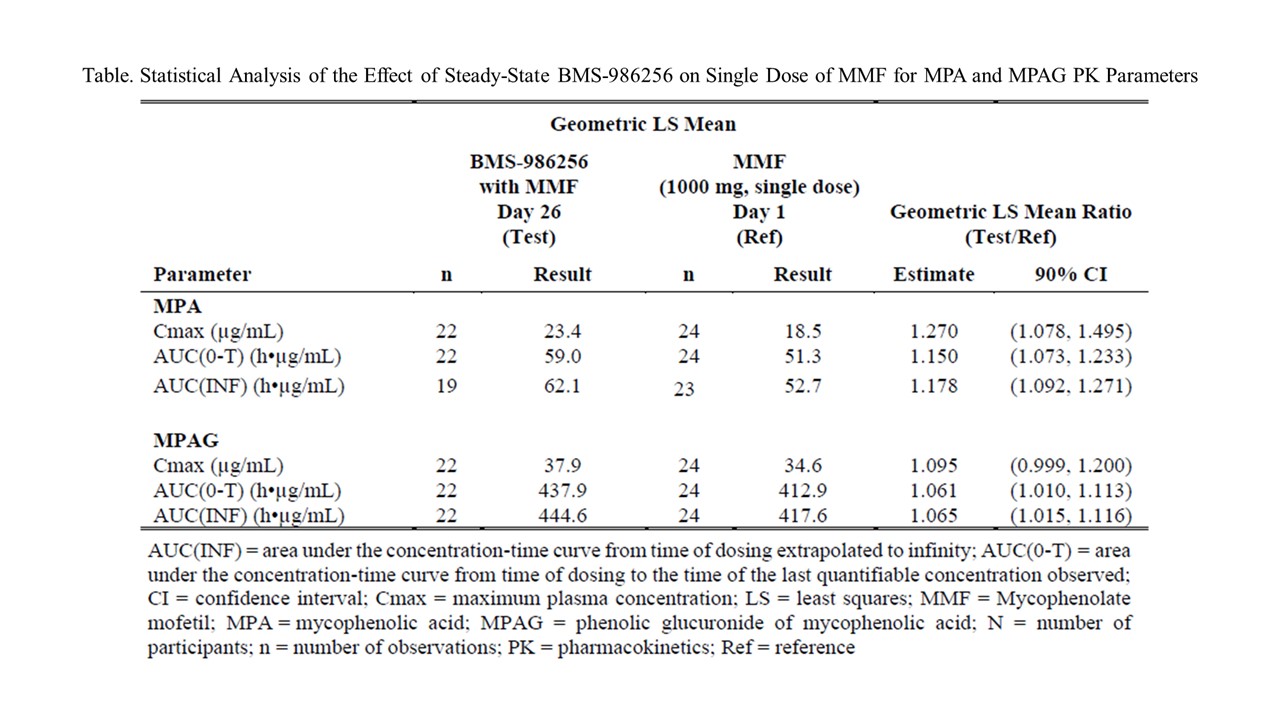
Results: Co-administration of BMS-986256 with MMF resulted in no clinically meaningful increase in exposures of MPA with geometric mean maximum concentration (Cmax) and total exposure (area under the curve extrapolated to infinity AUC(INF)) increased by 27 % and 17.8%, respectively, compared with MMF alone. Exposures of the MPA metabolite, MPAG, were comparable in both treatment groups, indicating no impact on metabolism of MPA by BMS-986256. In addition, single oral dose MMF did not alter steady state BMS-986256 and BMT-271199 (metabolite) exposures. BMS-986256 was generally safe and well-tolerated with and without MMF. There were no concerning abnormal laboratory test results, including tests of hepatic function and no serious AEs, deaths, or AEs leading to discontinuation. All treatment-emergent AEs were mild and resolved spontaneously.
Conclusion: BMS-986256 at steady state had no clinically meaningful effect on the PK of a single dose of MMF. MMF alone or in combination with BMS986256 was safe and well tolerated in this study. Given the lack of clinically meaningful DDI, MMF can be co-administered with BMS-986256 without any dose adjustment.
To cite this abstract in AMA style:
Chiney M, Girgis I, Harrison M, Zhang X, Shen Y, Dawes M, Dong L, Shevell D, Aras U, Murthy B. BMS-986256, an Oral Novel Toll-like Receptor 7 and 8 (TLR7/8) Inhibitor, Does Not Affect the Pharmacokinetics of Mycophenolate Mofetil in Healthy Subjects [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/bms-986256-an-oral-novel-toll-like-receptor-7-and-8-tlr7-8-inhibitor-does-not-affect-the-pharmacokinetics-of-mycophenolate-mofetil-in-healthy-subjects/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bms-986256-an-oral-novel-toll-like-receptor-7-and-8-tlr7-8-inhibitor-does-not-affect-the-pharmacokinetics-of-mycophenolate-mofetil-in-healthy-subjects/