Session Information
Date: Monday, November 9, 2020
Title: RA – Diagnosis, Manifestations, & Outcomes Poster IV: Lifespan of a Disease
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with RA exhibit wide variations in response to therapy. Early treatment response profiles may help us to better predict subsequent treatment response, thus expediting treatment optimization, improving outcomes, and reducing exposure to ineffective therapies. This exploratory study aimed to predict treatment outcomes to tofacitinib in patients with RA using hierarchical cluster analysis of baseline (BL) data, and cluster-specific regressions using BL data only vs BL data + early treatment response profiles.
Methods: This analysis included data from Phase 3 (NCT00814307; NCT00847613; NCT00856544; NCT00853385) and Phase 3b/4 (NCT02187055) randomized controlled trials (RCTs) of tofacitinib in patients with RA. Data were included from patients randomized to receive tofacitinib 5 mg twice daily (BID; monotherapy or + MTX/other csDMARDs) with ≥ 4 Clinical Disease Activity Index (CDAI) data points during 6 months of treatment and moderate/high BL CDAI severity; patients who discontinued the RCTs were assessed case-by-case for inclusion. A bottom-up hierarchical cluster analysis (using BL data) was conducted to improve prediction of tofacitinib response by identifying patient subgroups for cluster-specific regression models (using BL data ± early response profiles). The primary outcome was prediction of CDAI responder status at Month (M)6, using BL, M1, and M3 data, defined as achievement of minimum clinically important difference (MCID; CDAI change of 11/6/2 for patients with high/moderate/low BL CDAI severity); achievement of low disease activity (2.8< CDAI ≤ 10) or remission (CDAI ≤ 2.8) (ie treat-to-target approach for response) was also assessed. For cluster-specific regressions, patients of each cluster were randomized to a training (model development) or testing (model evaluation) dataset. The algorithm for outcome prediction consisted of all fixed BL demographics (eg age) ± time-lagged (M1 and M3) covariates (eg CDAI).
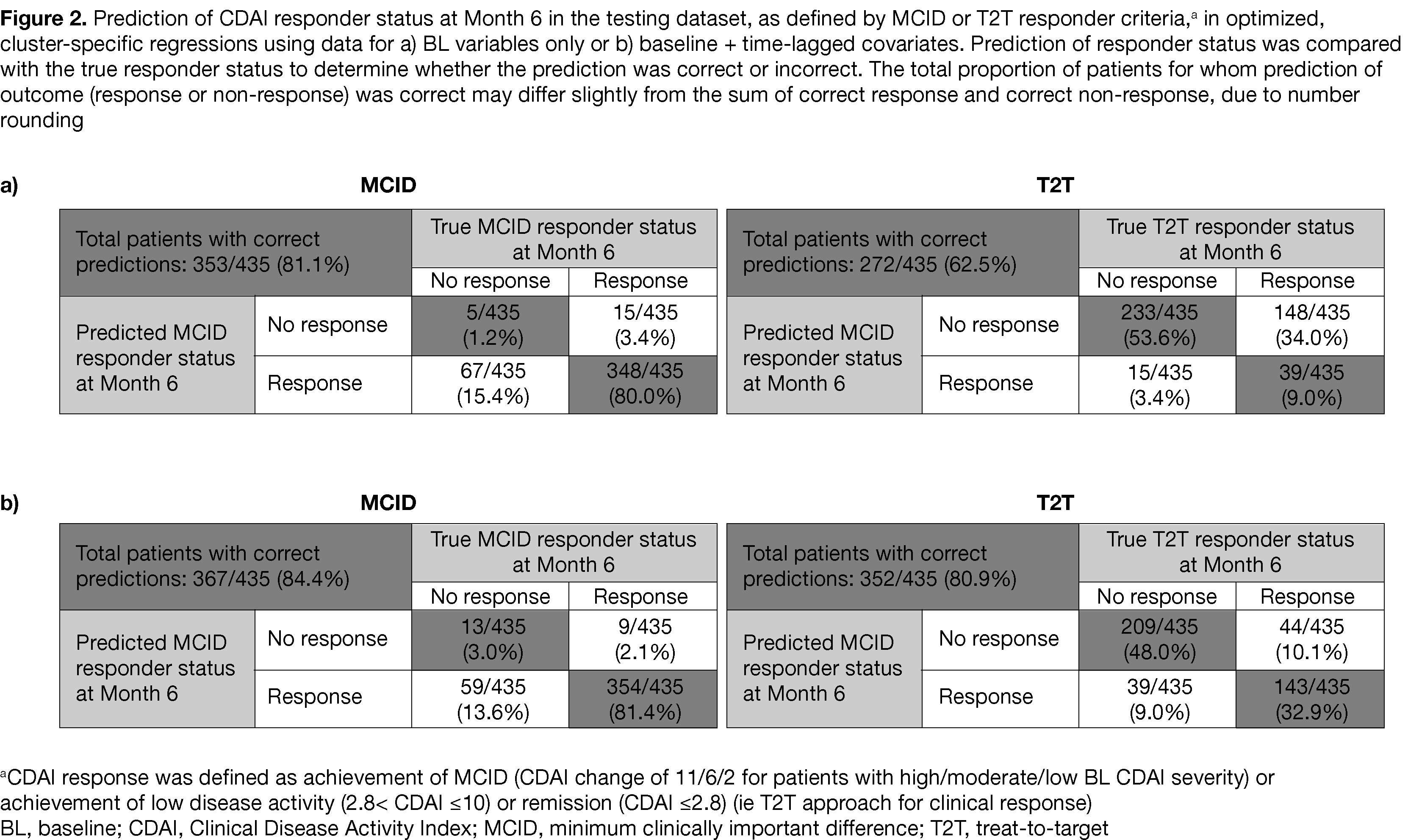
Results: Data from 1,617 patients with RA using tofacitinib 5 mg BID were included. Hierarchical cluster analysis using BL data had 6 clustering variables (tofacitinib monotherapy/combination therapy, prior corticosteroid use, smoking status, BL CDAI, BL HAQ-DI, BL pain discordance) and 5 unique clusters (ie subgroups; Figure 1). The training dataset (1,182 patients; 73.1%) was used to develop cluster-specific regressions which were then assessed in a testing dataset (435 patients; 26.9%). When regression models were used to predict outcome in the testing dataset using only BL data, prediction of MCID-defined CDAI responder status at M6 was correct for 81.1% of patients (Figure 2). Using BL + time-lagged covariates, prediction of MCID-defined CDAI responder status was correct for 84.4% of patients (Figure 2).
Conclusion: This hybrid of clustering and cluster-specific regressions, taking into account early treatment response profiles, is an interesting approach worthy of further exploration for predicting and improving long-term tofacitinib treatment outcomes in patients with RA.
Acknowledgments: Study sponsored by Pfizer Inc. Medical writing support was provided by Emma Deeks, CMC Connect, and funded by Pfizer Inc.
To cite this abstract in AMA style:
Landewé R, Solomon D, Bonfanti G, Manca L, Woolcott J, Deuring J, Watt S, Bhadra Brown P, Germino R, Emir B, Edwards R. Blending Hierarchical Cluster Analysis and Cluster-Specific Regressions to Predict Clinical Outcome to Tofacitinib Treatment in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/blending-hierarchical-cluster-analysis-and-cluster-specific-regressions-to-predict-clinical-outcome-to-tofacitinib-treatment-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/blending-hierarchical-cluster-analysis-and-cluster-specific-regressions-to-predict-clinical-outcome-to-tofacitinib-treatment-in-patients-with-rheumatoid-arthritis/