Session Information
Date: Sunday, November 12, 2023
Title: (0510–0542) Spondyloarthritis Including Psoriatic Arthritis – Treatment: AxSpA Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Bimekizumab (BKZ) is a monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A. In the phase 3 studies BE MOBILE 1 and 2, BKZ demonstrated sustained improvements to Week (Wk) 52 in patients (pts) with active non-radiographic (nr-) and radiographic axial spondyloarthritis (r-axSpA; i.e., AS).1,2
Here, we report the impact of BKZ in pts with axSpA on key pt-reported symptoms (spinal pain, stiffness, and fatigue).
Methods: BE MOBILE 1 (NCT03928704) and 2 (NCT03928743) both comprised a 16-wk double-blind period followed by a 36-wk maintenance period. Pts were randomized to receive subcutaneous BKZ 160 mg every 4 wks (Q4W) or placebo (PBO); from Wk 16 all pts received BKZ 160 mg Q4W.
We report mean nocturnal and total spinal pain, and BASDAI morning stiffness (mean of BASDAI questions 5 and 6), as well as mean change from baseline (CfB) in Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scores to Wk 52; missing values were imputed using multiple imputation. Least squares mean difference (LSMD) is reported for FACIT-F at Wk 16. Proportion of pts achieving low levels of pain (total and nocturnal spinal pain score: ≤2 and ≤4) and a meaningful improvement in fatigue (FACIT-F score: ≥8 point increase from baseline [BL]), analyzed post hoc, are also reported; missing values were imputed using non-responder imputation.
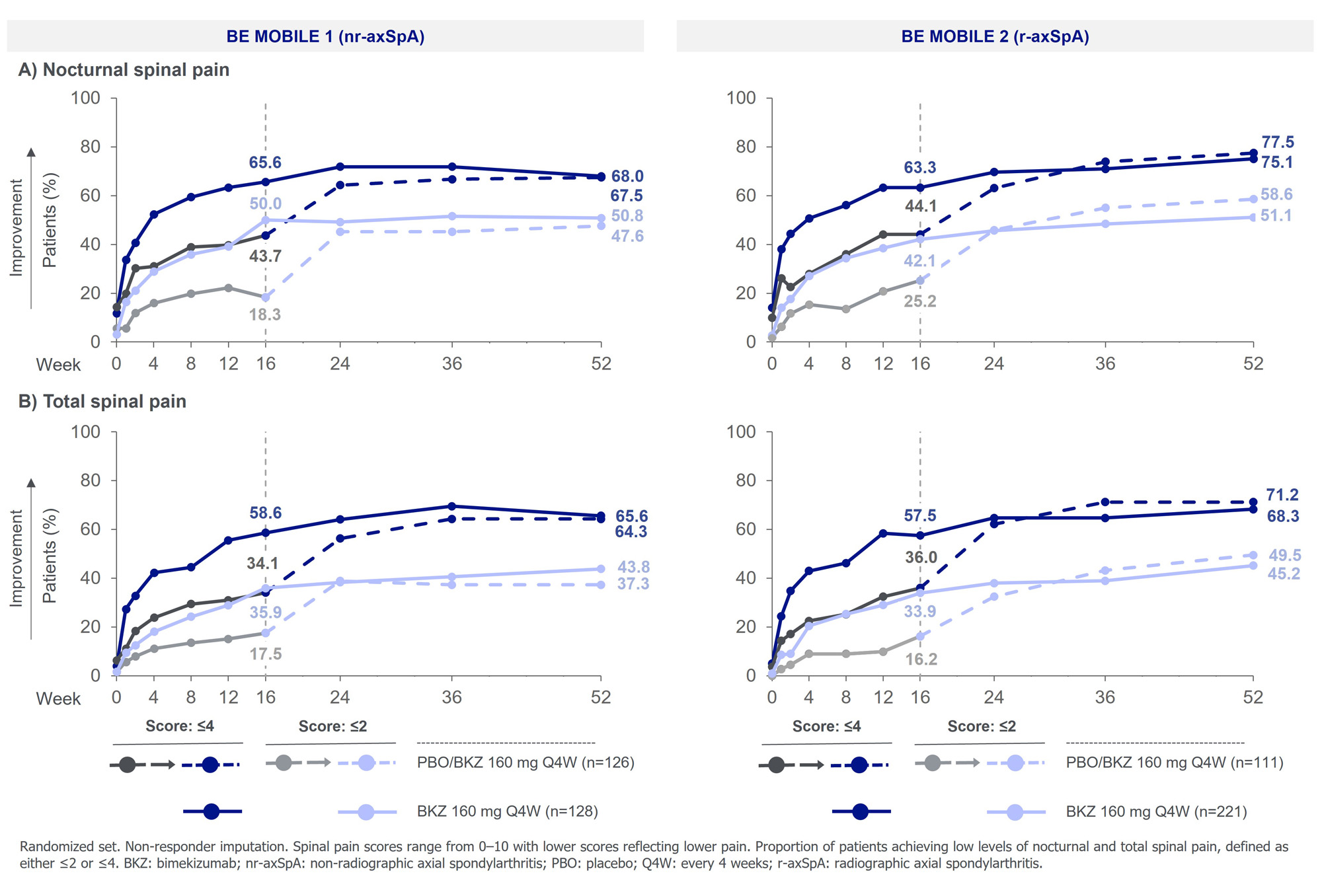
Results: 254 pts with nr-axSpA (BKZ: 128; PBO: 126) and 332 with r-axSpA (BKZ: 221; PBO: 111) were randomized; 86.6% (220/254) and 89.8% (298/332) pts completed to Wk 52, respectively. Across both studies, mean BL scores for all reported outcomes indicated high disease burden (Figure 1).
At Wk 16, BKZ-randomized pts achieved greater improvements in mean nocturnal and total spinal pain (nominal), and BASDAI morning stiffness (nominal) vs PBO (all p< 0.001; Figure 1). Mean scores were further improved to Wk 52 among BKZ-randomized pts and among pts who switched from PBO to BKZ at Wk 16 (PBO/BKZ), responses approached those of BKZ-randomized pts. Similarly, at Wk 16 a higher proportion of BKZ- vs PBO-randomized pts achieved low nocturnal and total spinal scores (Figure 2); at Wk 52 these improvements were similar across BKZ-randomized and PBO/BKZ pts.
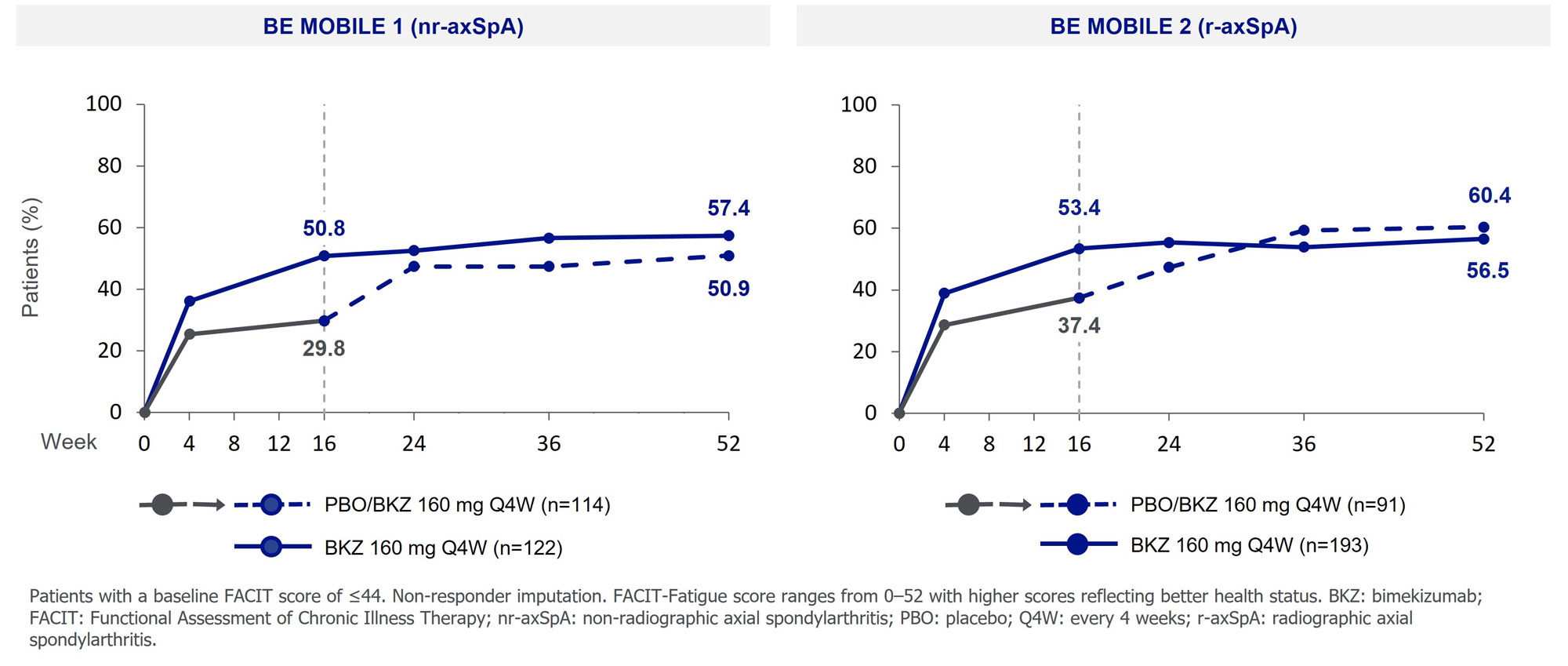
At Wk 16, BKZ-randomized pts achieved greater improvements in FACIT-F scores vs PBO (mean CfB [nominal p value]: nr-axSpA: 8.5 vs 3.9 [< 0.001]; r-axSpA: 8.4 vs 5.0 [0.015]; LSMD: nr-axSpA: 4.2; r-axSpA: 2.2) with similar improvements at Wk 52 among BKZ-randomized and PBO/BKZ pts (mean CfB: nr-axSpA: 10.9 vs 9.2; r-axSpA: 9.9 vs 9.5). Similarly, a higher proportion of BKZ-randomized pts achieved a ≥8 point improvement from BL compared with PBO at Wk 16 (Figure 3);responses at Wk 52 were similar across PBO/BKZ and BKZ-randomized pts.
Conclusion: BKZ treatment resulted in clinically meaningful improvements in spinal pain, morning stiffness, and fatigue to Wk 52 in pts across the full disease spectrum of axSpA, who had a similar and high disease burden at BL. These findings emphasize the benefit of BKZ on clinical symptoms which are important to pts and have a substantial impact on their daily lives.3
References:1. van der Heijde D. Ann Rheum Dis 2023;82:515–526; 2. Boel A. Ann Rheum Dis. 2019;78:1545–9; 3. Strand V. J Clin Rheumatol 2017;23:383–91.
To cite this abstract in AMA style:
Mease P, Dougados M, Dubreuil M, Magrey M, Marzo-Ortega H, Rudwaleit M, de la Loge C, Fleurinck C, Massow U, Taieb V, Deodhar A. Bimekizumab Treatment Improved Key Patient-Reported Symptoms of Axial Spondyloarthritis Including Spinal Pain, Fatigue, and Morning Stiffness: 52-Week Results from Two Phase 3 Studies [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/bimekizumab-treatment-improved-key-patient-reported-symptoms-of-axial-spondyloarthritis-including-spinal-pain-fatigue-and-morning-stiffness-52-week-results-from-two-phase-3-studies/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bimekizumab-treatment-improved-key-patient-reported-symptoms-of-axial-spondyloarthritis-including-spinal-pain-fatigue-and-morning-stiffness-52-week-results-from-two-phase-3-studies/