Session Information
Date: Sunday, November 12, 2023
Title: (0510–0542) Spondyloarthritis Including Psoriatic Arthritis – Treatment: AxSpA Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Axial spondyloarthritis (axSpA) severely impairs physical function and health-related quality of life (HRQoL).1 Bimekizumab (BKZ), a monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A, met all primary and ranked secondary endpoints at Week (Wk) 16 in patients (pts) with axSpA in the phase 3 studies BE MOBILE 1 and 2; efficacy was sustained to Wk 52.2
Here, we assess the impact of BKZ on physical function and HRQoL in pts with axSpA to Wk 52.
Methods: BE MOBILE 1 (NCT03928704; non-radiographic [nr-] axSpA) and 2 (NCT03928743; radiographic [r-] axSpA; i.e., AS)3 were randomized, placebo (PBO)-controlled trials comprising a 16-wk double-blind period followed by a 36-wk maintenance period. Pts were randomized to subcutaneous BKZ 160 mg every 4 wks (Q4W) or PBO; from Wk 16 PBO-randomized pts switched to BKZ 160 mg Q4W (PBO/BKZ). Physical function was assessed by BASFI (0–10) and HRQoL by AS quality of life questionnaire (ASQoL; 0–18).
We report mean BASFI and ASQoL scores to Wk 52 with multiple imputation (MI), and proportion of pts achieving low BASFI score (≤2) and clinically relevant ASQoL improvement (≥4-point reduction from baseline [BL])4 with non-responder imputation. Proportion of BKZ-randomized pts impacted by each ASQoL item (i.e., responded “yes”) to Wk 52 is reported as observed.
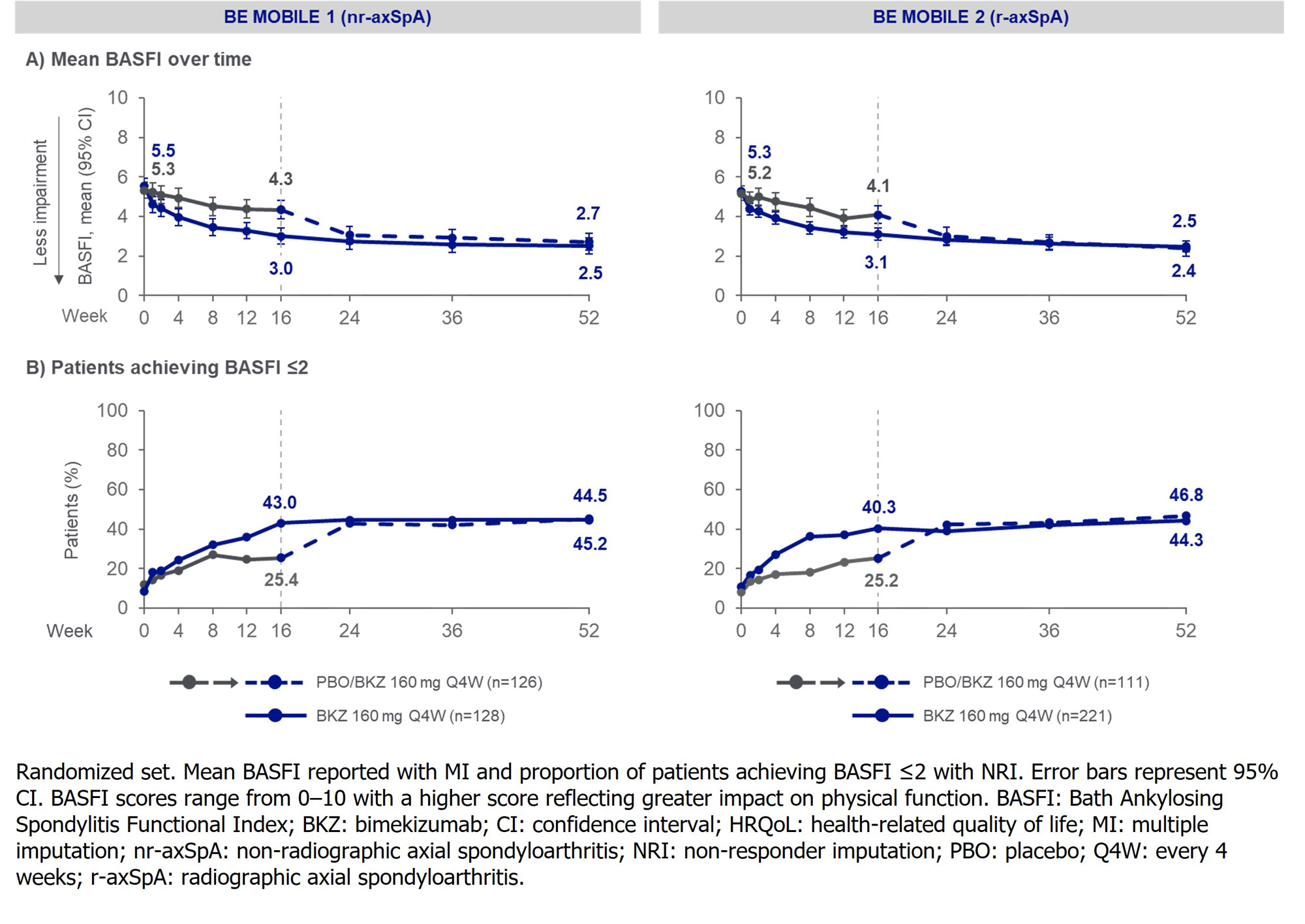
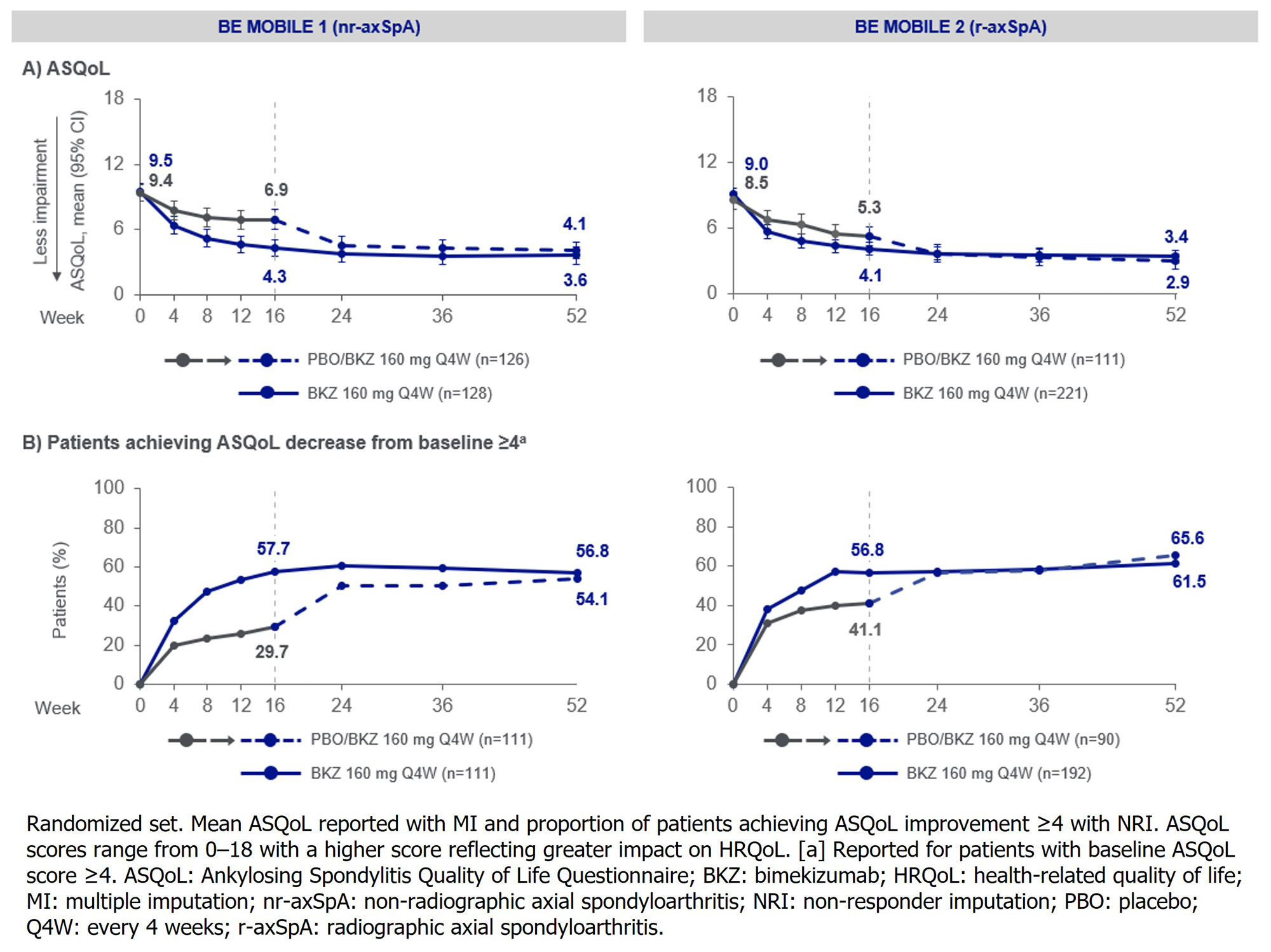
Results: 254 pts with nr-axSpA (BKZ: 128; PBO: 126) and 332 with r-axSpA (BKZ: 221; PBO: 111) were randomized. Mean BL BASFI and ASQoL indicated impaired physical function and HRQoL (Figure 1A, 2A).
At Wk 16, significantly greater reductions from BL (i.e., improvement) in BASFI and ASQoL were observed in BKZ-treated pts vs PBO (p< 0.001; reference-based MI). Mean scores remained low or decreased at Wk 52 in both BKZ-randomized vs PBO/BKZ pts for BASFI (nr-axSpA: 2.5 vs 2.7; r-axSpA: 2.5 vs 2.4) and ASQoL (3.6 vs 4.1; 3.4 vs 2.9; Figure 1A, 2A).
A higher proportion of BKZ- vs PBO-treated pts achieved BASFI ≤2 and ≥4 ASQoL reduction from BL at Wk 16; these proportions were sustained or improved to Wk 52, and were similar among BKZ-randomized vs PBO/BKZ pts for BASFI ≤2 (nr-axSpA: 44.5% vs 45.2%; r-axSpA: 44.3% vs 46.8%) and ≥4 ASQoL reduction from BL (56.8% vs 54.1%; 61.5% vs 65.6%; Figure 1B, 2B).
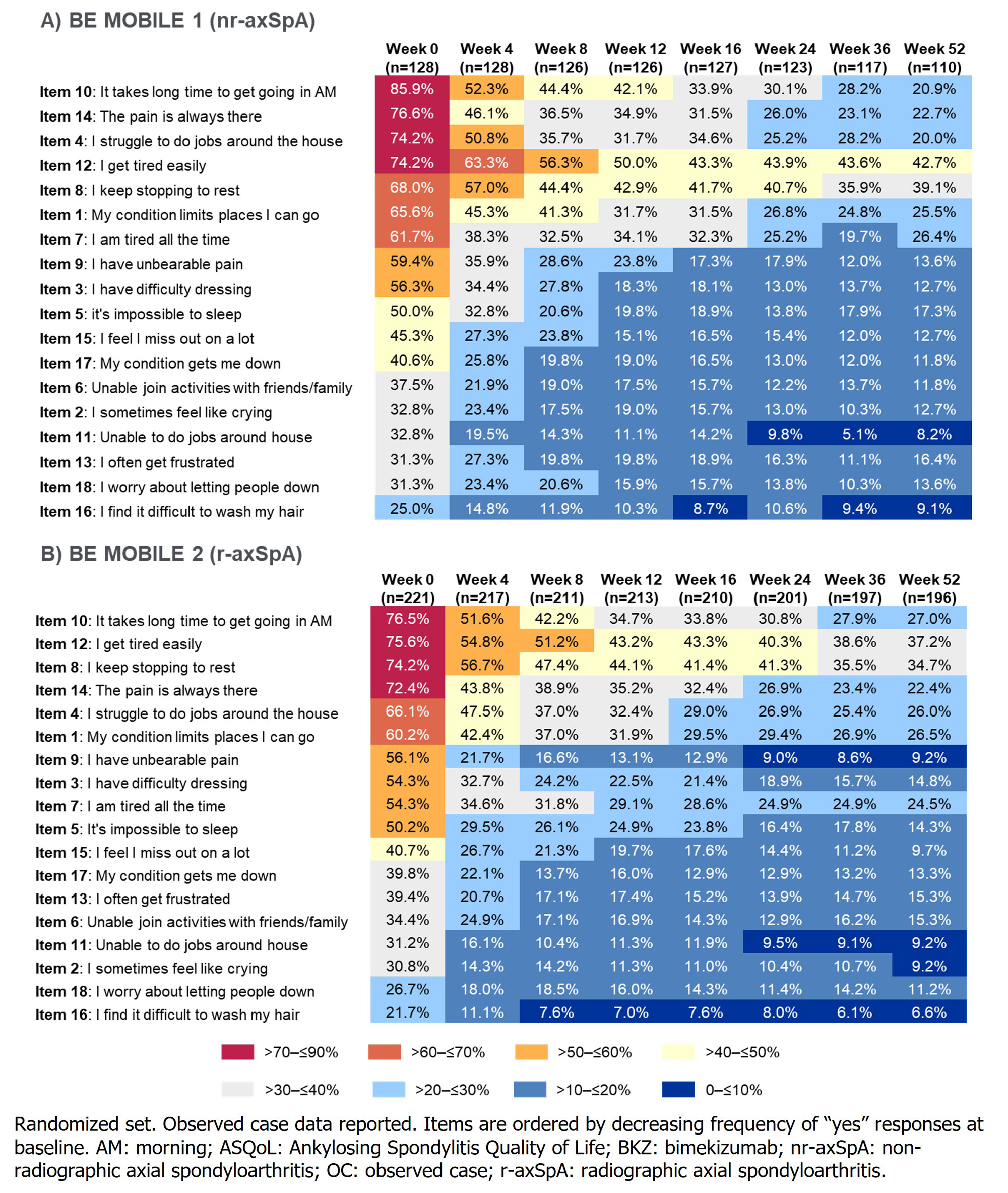
ASQoL items impacting ≥66% of BKZ-randomized pts with nr-axSpA and r-axSpA at BL were items 10 (it takes a long time to get going in the morning; 85.9% and 76.5%), 14 (pain is always there; 76.6% and 72.4%), 12 (I get tired easily; 74.2% and 75.6%), 4 (I struggle to do jobs around the house; 74.2% and 66.1%), and 8 (I keep stopping to rest; 68.0% and 74.2%). Reductions from BL were seen in the proportion of pts responding “yes” to each ASQoL item through 52 wks of BKZ treatment; the greatest reductions at Wk 52 were in items 10 (nr-axSpA: –65.0%; r-axSpA: –49.5%) and 14 (–53.9%; –50.0%; Figure 3).
Conclusion: BKZ resulted in high proportions of pts achieving improvements in physical function and HRQoL through 52 wks of treatment across the full disease spectrum of axSpA.
References:1. Strand V. J Clin Rheumatol 2017;23:383–91; 2. Baraliakos X. Arthritis Rheumatol 2022;74(suppl 9); 3. Boel A. Ann Rheum Dis 2019;78:1545–9; 4. Hoepken B. Qual Life Res. 2021;30:945–54.
To cite this abstract in AMA style:
Dubreuil M, Gaffney K, Kay J, Navarro-Compán V, de la Loge C, Ellis A, Fleurinck C, Massow U, Taieb V, Deodhar A. Bimekizumab Improved Physical Function and Health-Related Quality of Life in Patients with Axial Spondyloarthritis: 52-Week Results from Two Phase 3 Studies [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/bimekizumab-improved-physical-function-and-health-related-quality-of-life-in-patients-with-axial-spondyloarthritis-52-week-results-from-two-phase-3-studies/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bimekizumab-improved-physical-function-and-health-related-quality-of-life-in-patients-with-axial-spondyloarthritis-52-week-results-from-two-phase-3-studies/