Session Information
Date: Saturday, November 16, 2024
Title: B Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The dramatic demonstration of CD19 CAR-T efficacy in systemic lupus erythematosus (SLE), idiopathic inflammatory myositis, and systemic sclerosis by Georg Schett and colleagues (F. Muller et al., N Engl J Med 2024 Feb 22;390(8):687-700) has opened the possibility that autoimmunity in such diseases may be reset through the depletion of B cells leading to durable remissions. Given the challenges of deploying CAR-T at large scale and in a diverse patient population whose disease severity varies considerably, there is greatly renewed interest in next-generation NK and T cell engagers to safely achieve deep depletion of autoantibody producing cells.
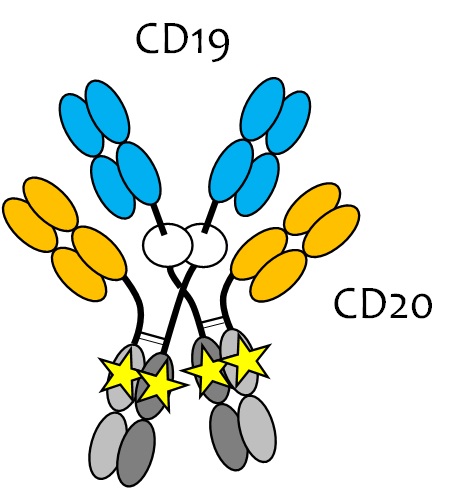
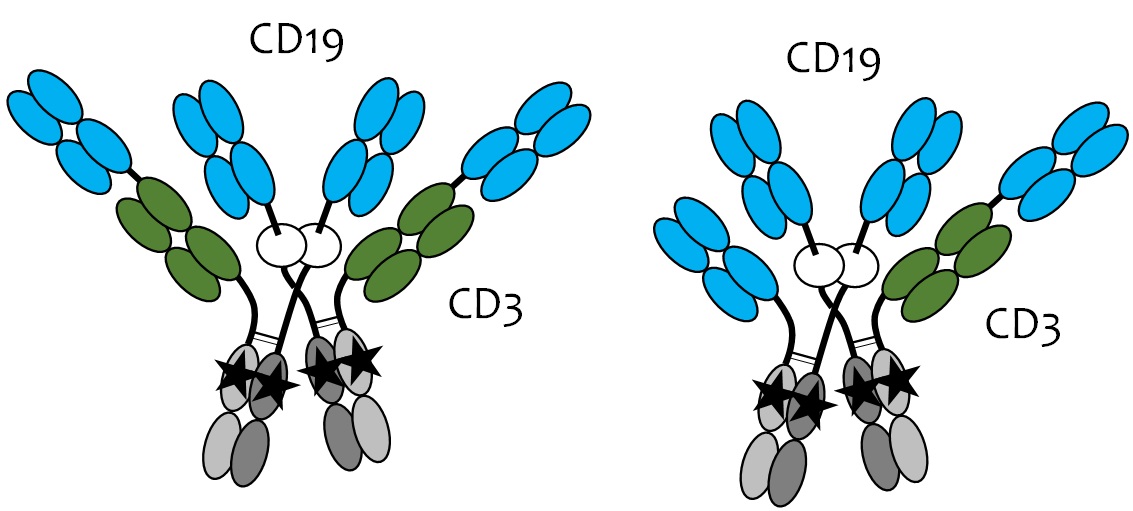
Methods: We describe a new approach called topological engineering (GEM-DIMERTM technology) allowing us to create classes of superdimeric, tetrahedral antibodies demonstrating cooperative binding to target and effector cells. The first class (Fig.1) is illustrated by a novel multivalent NK/monocyte engager (i.e., CD16a engager) comprising two anti-CD19 (huFMC63) and two anti-CD20 (rituximab) target binding domains, and two Fc effector binding domains incorporating the S239D/I332E double mutation enhancing Fcg receptor binding. A second class (Fig.2) is illustrated by novel multivalent T cell engagers (i.e., CD3 engager) comprising four anti-CD19 or anti-FolRα target binding domains, one or two anti-CD3 effector binding domains, and two silenced Fc domains. T cell engagers under evaluation include anti-CD19 target cell binding domains derived from either tafasitamab or FMC63, the anti-CD19 targeting moiety in the CAR-T products YescartaTM and KymriaTM.
Results: CD19/CD20 dual-targeting GEM-DIMER NK/monocyte engagers demonstrated enhanced binding to target and effector cells, as well as increased induction of apoptosis, ADCC, and ADCP compared against the parent antibodies. We observe a dramatic increase in binding to low affinity Fcg receptors (e.g., FcgRIIIa CD16a/158V and CD16a/158F) of two to three orders of magnitude. In contrast, binding to the high affinity Fcg receptor (FcgRI CD64) was modestly increased, indicating a significant shift in Fcg receptor binding specificity in favor of effectors such as NK cells and monocytes expressing such low affinity Fcg receptors. Multivalent FolRα-targeting GEM-DIMER T cell engagers with four anti-FolRα domains demonstrated enhanced binding to FolRα-expressing IGROV-1 target cells of up to one order of magnitude greater than a FolRα-targeting 2+1 T cell engager with only two anti-FolRα domains, indicating increased specificity and presumed safety for the disease target.
Conclusion: The ability of GEM-DIMER NK/monocyte engagers to potently and selectively engage low affinity FcgR-expressing cells such as NK cells and monocytes, and the ability of GEM-DIMER T cell engagers to bind more selectively to disease targets offers new opportunities beyond those possible with conventional antibodies and CAR-T. CD19/CD20 and CD19 targeting tetravalent GEM-DIMER NK/monocyte engagers and T cell engagers demonstrating cooperative binding to disease targets and effector cells are promising candidates for broad and deep depletion of B cells with reduced risk of re-emergence of autoimmune-reactive variants.
To cite this abstract in AMA style:
Capon D, Troitskaya L, Fomin M, Frank B, Edman U, Capon B, Law B, Chapin S, Lewis G, Gefter M, Punnonen J, Chan N. Beyond Antibodies and CAR-T: Topologically Engineered, Superdimeric Antibody NK Engagers and T Cell Engagers for B Cell Depletion Demonstrating Cooperative Binding to Target and Effector Cells [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/beyond-antibodies-and-car-t-topologically-engineered-superdimeric-antibody-nk-engagers-and-t-cell-engagers-for-b-cell-depletion-demonstrating-cooperative-binding-to-target-and-effector-cells/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/beyond-antibodies-and-car-t-topologically-engineered-superdimeric-antibody-nk-engagers-and-t-cell-engagers-for-b-cell-depletion-demonstrating-cooperative-binding-to-target-and-effector-cells/