Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The ADVOCATE trial evaluated avacopan (a C5a receptor antagonist) as a replacement for a standard prednisone taper in the treatment of granulomatosis with polyangiitis (GPA) or microscopic polyangiitis (MPA), two types of ANCA-associated vasculitis. ADVOCATE used the Glucocorticoid Toxicity Index (GTI) to measure the change in glucocorticoid (GC) toxicity during the trial. Patients’ baseline GC-related toxicity, which may serve as an important predictor of clinical outcomes and future GC toxicity, has not been reported. This study aimed to evaluate patients’ GC-related toxicity at baseline in the ADVOCATE trial, using an adaptation of the GTI—the GT-SNAPSHOT—that assesses patients’ GC toxicity at a single point in time.
Methods: This analysis evaluated the overall GT-SNAPSHOT scores among all patients in the ADVOCATE trial at baseline. This post-hoc analysis reports the overall and individual GT-SNAPSHOT domain scores of those with newly-diagnosed versus relapsing disease.
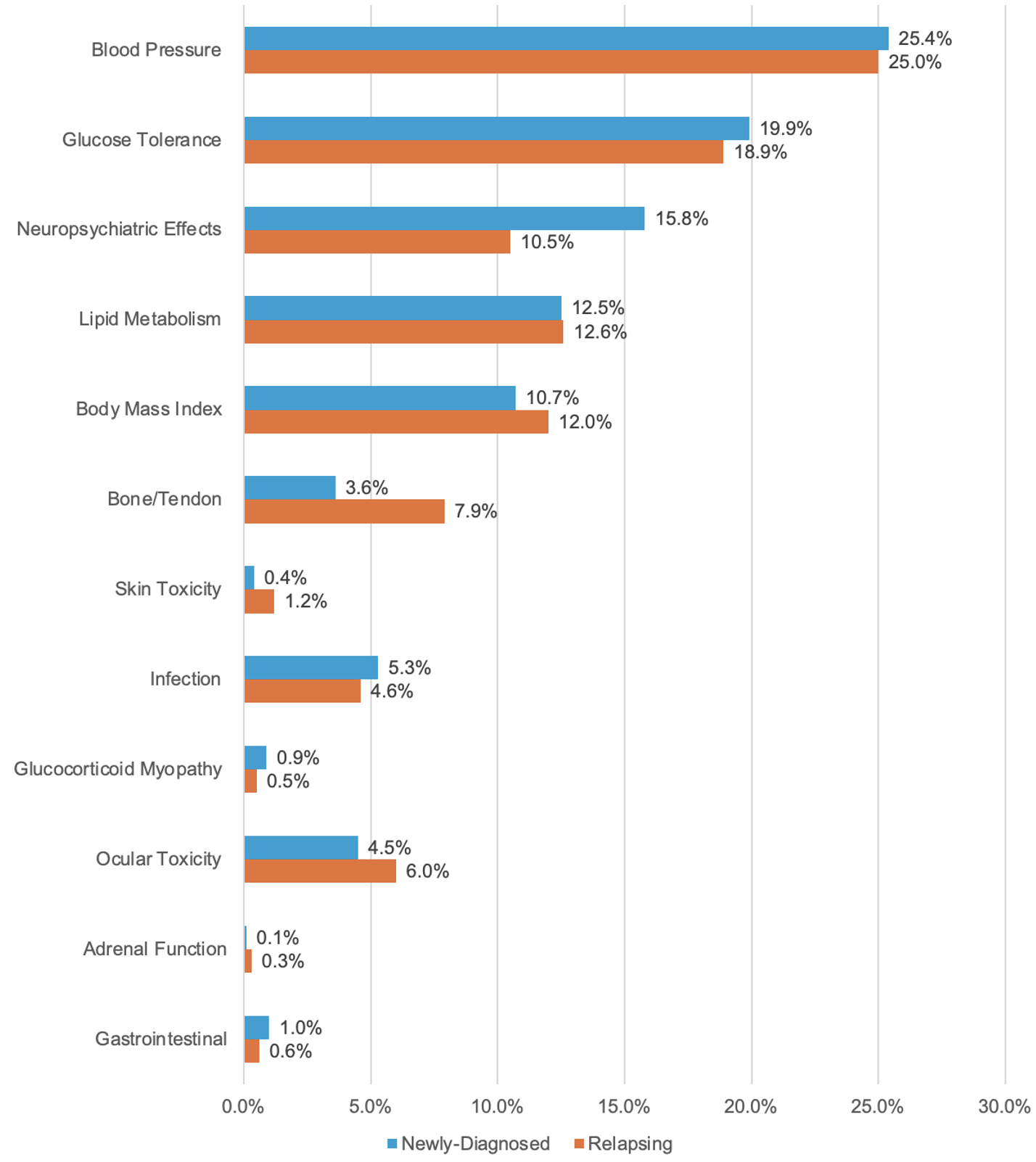
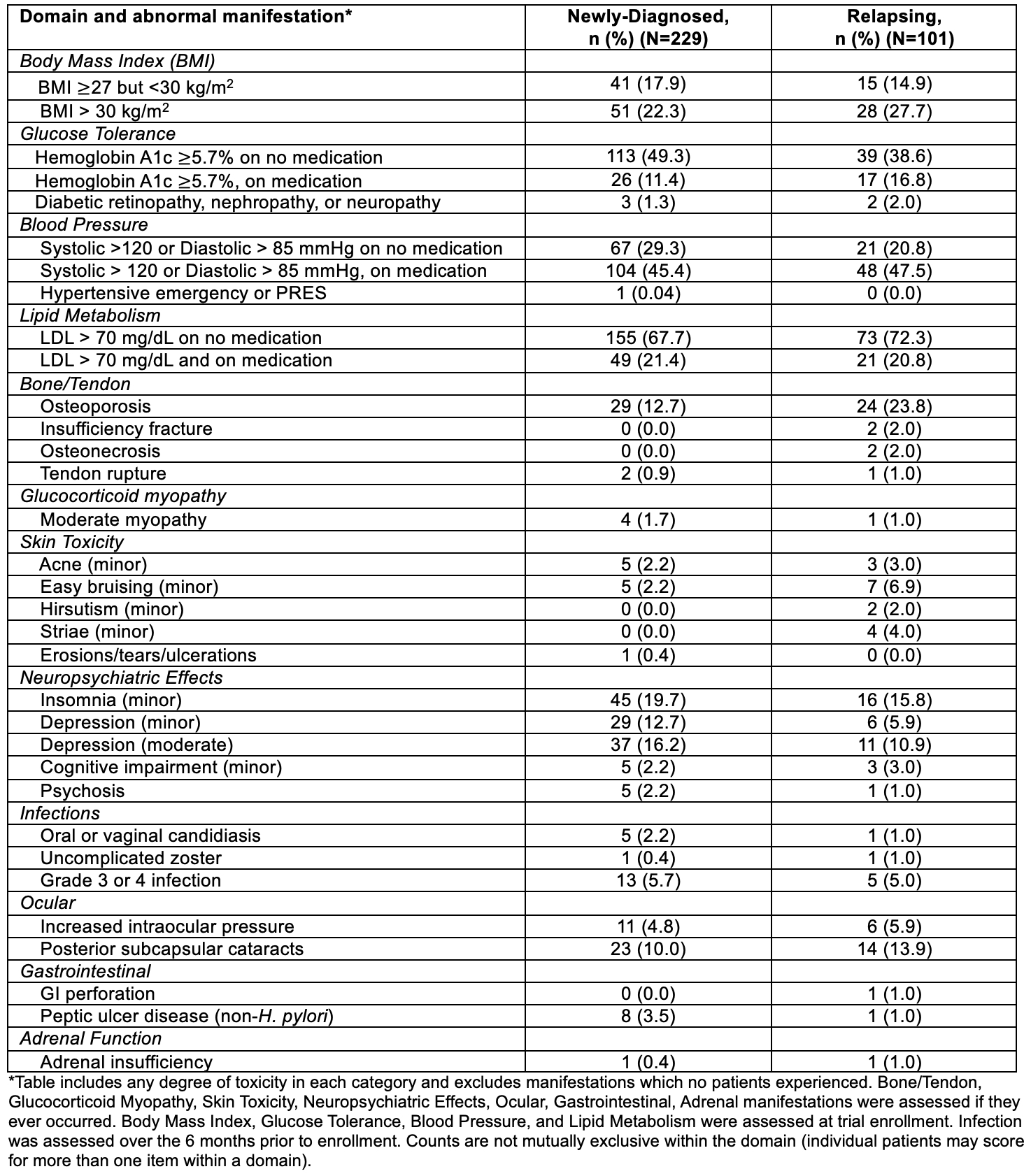
Results: Of the 330 patients in ADVOCATE, 229 had newly-diagnosed GPA or MPA, and 101 had relapsing GPA or MPA. The GT-SNAPSHOT domains that contributed the most to the overall scores were the Blood Pressure, Glucose Tolerance, Neuropsychiatric Effects, and Lipid Metabolism domains (Figure). GT-SNAPSHOT scores were similar in those with newly-diagnosed vs. relapsing disease (median [IQR] of 102 [65-137] vs 98 [68-142], respectively). Patients with relapsing disease were more likely to have obesity (BMI >30 kg/m2), hemoglobin A1c ≥ 5.7% on anti-hyperglycemic medication, osteoporosis, and ocular toxicity (cataracts or increased intraocular pressure). Those with newly-diagnosed disease had more neuropsychiatric manifestations, specifically insomnia and depression, and peptic ulcer disease. Infections prior to baseline were roughly balanced between groups (Table 1).
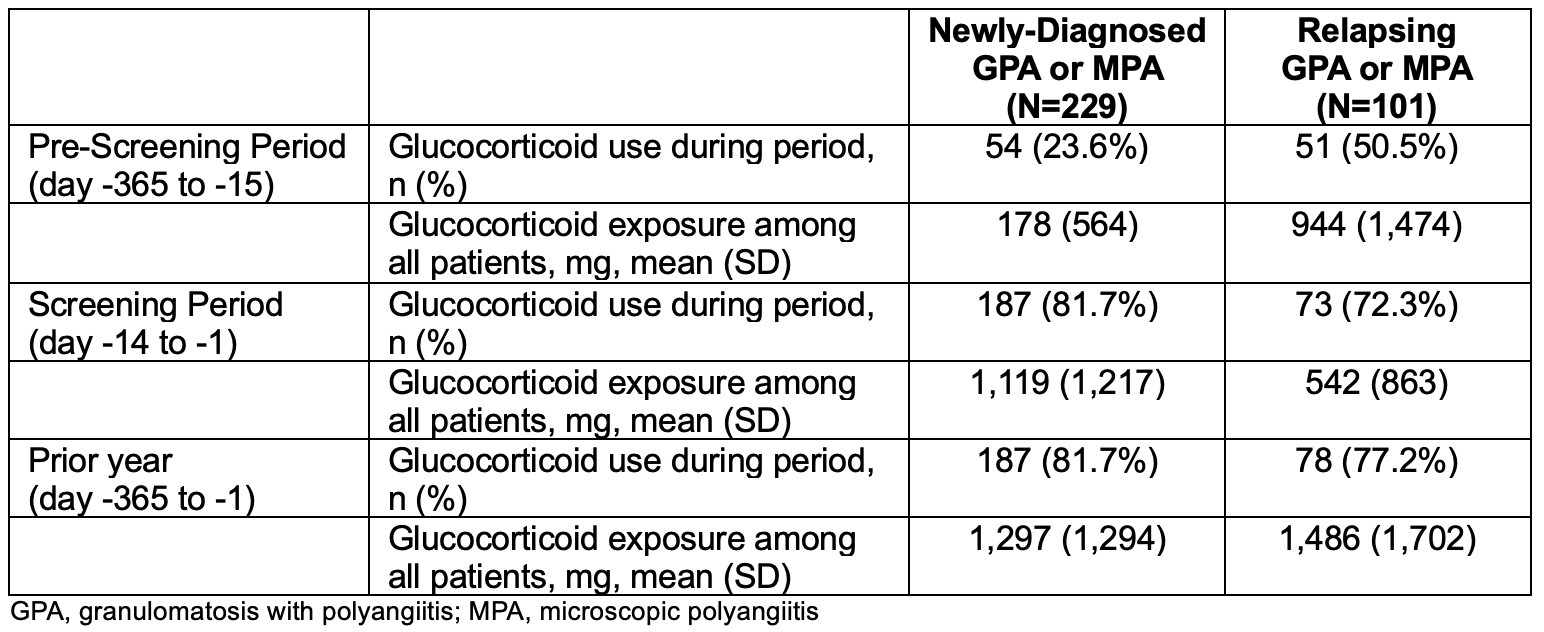
The finding of similar GT-SNAPSHOT scores in those with newly-diagnosed vs. relapsing disease prompted evaluation of their GC use prior to baseline. The mean cumulative GC exposure in the year prior to baseline was similar between the two groups overall (1,297 vs. 1,486 mg, respectively), but there were differences in pattern and timing of GC use (Table 2). Newly-diagnosed patients had higher mean cumulative GC exposure during screening (encompassing 14 days prior to baseline) compared to those with relapsing disease: 1,119 vs. 542 mg, respectively. In contrast, patients with relapsing disease had higher cumulative exposure in the 351 days before screening: 944 vs. 178 mg, respectively.
Conclusion: In ADVOCATE, patients with newly-diagnosed GPA or MPA had GT-SNAPSHOT scores similar to those with relapsing disease, indicating comparable baseline GC-related toxicity. However, the affected GC toxicity domains differed between groups, with GC toxicities associated with chronic GC exposure more commonly seen among patients with relapsing disease and GC toxicities that can develop more rapidly more commonly seen in those with newly-diagnosed disease.
To cite this abstract in AMA style:
Patel N, Bray S, Trimpe D, Jayne D, Merkel P, Stone J. Baseline Glucocorticoid-Related Toxicity in Newly-Diagnosed and Relapsing ANCA-Associated Vasculitis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/baseline-glucocorticoid-related-toxicity-in-newly-diagnosed-and-relapsing-anca-associated-vasculitis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baseline-glucocorticoid-related-toxicity-in-newly-diagnosed-and-relapsing-anca-associated-vasculitis/