Session Information
Date: Monday, November 8, 2021
Title: RA – Treatments Poster II: PROs, Biomarkers, & Systemic Inflammation (1223–1256)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Predictive biomarkers reflecting RA processes and treatment (tmt) efficacy are urgently needed to inform medical options. Markers of bone remodeling and extracellular matrix (ECM) turnover may serve as disease-relevant, surrogate biomarkers indicative of synovial joint pathophysiology.1,2 We evaluated baseline (BL) correlations of biomarkers with disease activity (DA), their predictive value for efficacy, and pharmacodynamic (PD) changes during subcutaneous (SC) abatacept (ABA) tmt in a phase 3b study in MTX-naive, ACPA+ patients (pts) with early RA (AVERT-2; NCT02504268).3
Methods: Pts with early RA (ACR/EULAR 2010)4 were randomized to SC ABA 125 mg QW or SC ABA placebo (PBO) both with oral MTX for 56 weeks (wks). A subgroup of the overall population (intention-to-treat) was included. Linear correlations were assessed using BL levels of 8 ECM serum biomarkers (Nordic Biosciences) including MMP-mediated degradation products of type III and IV collagen (C3M, C4M), neo-epitope of MMP-mediated degradation of CRP (CRPM), and neo-epitope of cathepsin-mediated degradation of type I collagen (CTX-I), with DA measures. Logistic regression predictive models were used to evaluate BL bone remodeling markers in predicting clinical efficacy (SDAI remission, DAS28 [CRP] < 2.6, ACR70) at wks 24 and 52 with biomarker levels defined as a continuous variable or categorically (low, medium, high tertiles). For biomarkers with data at wks 24 and 52, PD changes were assessed using a linear mixed-effects model with repeated measures.
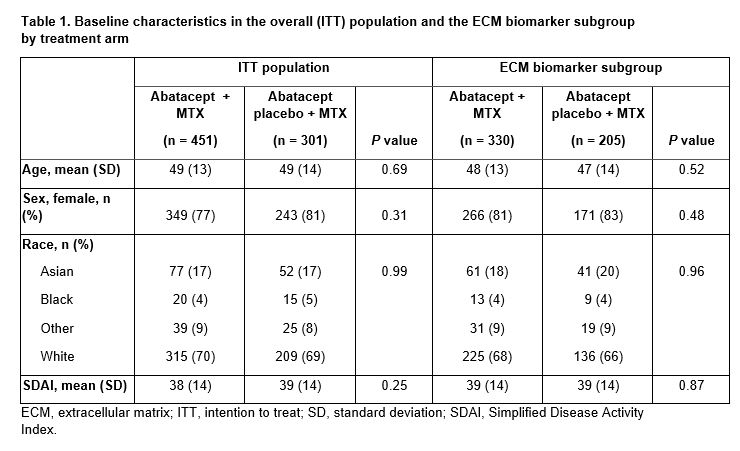
Results: BL pt characteristics were similar between the overall population and the ECM biomarker subgroup, and across tmt arms (Table 1). BL biomarker levels were generally similar between tmt arms. Of biomarkers tested, BL C3M, C4M, and CRPM showed greatest correlations with BL DA measures; CTX-I showed weak correlation with BL DA. Probability of wk 24 and 52 efficacy as predicted by BL CTX-I showed differential response between ABA + MTX and ABA PBO + MTX tmt arms at both time points (Figure 1). This was not seen with BL CRP (data not shown). Categorical analysis showed greater tmt differences for ABA + MTX vs ABA PBO + MTX in pts with medium/high vs low BL CTX-I (Figure 2). PD analysis showed significant tmt differences in adjusted change from BL in C3M and C4M (wk 24 and 52) and CRPM (wk 24) with ABA + MTX vs ABA PBO + MTX (data not shown).
Conclusion: In ACPA+ pts with early RA, BL levels of cartilage biomarker CTX-I predicted differential SDAI remission or DAS28 (CRP) < 2.6 with abatacept + MTX vs abatacept PBO + MTX, with higher levels of CTX-I associated with a greater probability of achieving efficacy endpoints for abatacept + MTX; this relationship was not observed with abatacept PBO + MTX. Tmt differences were greatest in pts with medium/high BL CTX-I levels. Significant PD effects of abatacept tmt on C3M, C4M, and CRPM were seen. Additional studies of BL CTX-I as a predictive biomarker of abatacept tmt response are warranted.
References:
1. Gudmann NS, et al. Clin Exp Rheumatol 2018;36:462–470.
2. Blair JPM, et al. PLoS ONE 2019;14:e0219980.
3. Emery P, et al. Arthritis Rheum 2018;70:abs563.
4. Aletaha D, et al. Arthritis Rheum 2010;62:2569–81.
Medical writing: Joanna Wright (Caudex), funded by Bristol Myers Squibb
To cite this abstract in AMA style:
Emery P, Tanaka Y, Bykerk V, Bingham C, Huizinga T, Citera G, Wu C, Hu S, Schafer P, Liu J, Connolly S, Wong R, Fleischmann R. Baseline Extracellular Matrix Biomarkers Predict Abatacept Treatment Response in MTX-Naive, ACPA+ Patients with Early RA [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/baseline-extracellular-matrix-biomarkers-predict-abatacept-treatment-response-in-mtx-naive-acpa-patients-with-early-ra/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baseline-extracellular-matrix-biomarkers-predict-abatacept-treatment-response-in-mtx-naive-acpa-patients-with-early-ra/