Session Information
Date: Monday, November 14, 2022
Title: RA – Treatment Poster IV
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Deterioration of peripheral bone mass and bone biomechanics are well-known consequences of Rheumatoid Arthritis (RA)[1]. CCP-AB pos. RA, in particular, leads to a significant decline in functional properties of bone which is also associated with an increased fracture risk[2]. Bone biomechanics such as bone strength and fragility can be measured by micro-finite element analysis (µFEA) allowing to nondestructively quantify the resistance of bone to stress and strain based on high-resolution CT data (HR-pQCT) [3]. Preclinical and clinical observations suggest a positive effect of JAK inhibitors on bone mass and microstructure. However, no prospective interventional clinical study has evaluated bone strength in RA [4].The aim of this study is to evaluate the changes in bone strength in patients with active RA under baricitinib treatment using µFEA on the radial bone.
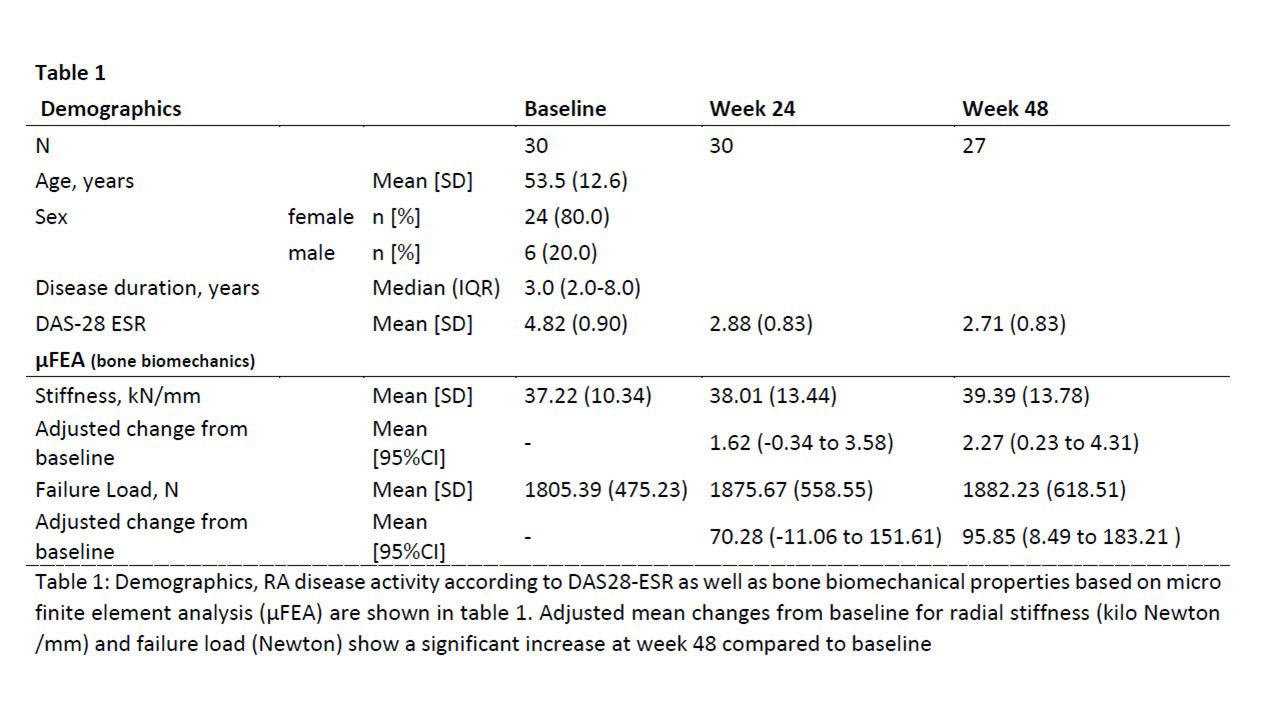
Methods: BAREBONE is a prospective, open label, interventional, single-center study (EUDRACT 2018-001164-32/NCT03701789) to assess peripheral bone density, microstructure and bone biomechanical properties in patients with active RA treated with BARI (4mg/day). HR-pQCT scans of the radius were performed at baseline, week 24 and week 48. Bone biomechanical properties (resistance of the bone in kilo Newton/kN /mm) and bone strength as estimated failure load (Newton /N) were estimated using µFEA analysis. We report descriptive data at study visits and analyzed change from baseline in stiffness and failure load using linear mixed effects models adjusting for age and sex and linear regression to explore association of density and microstructural changes with changes in stiffness and failure load from baseline.
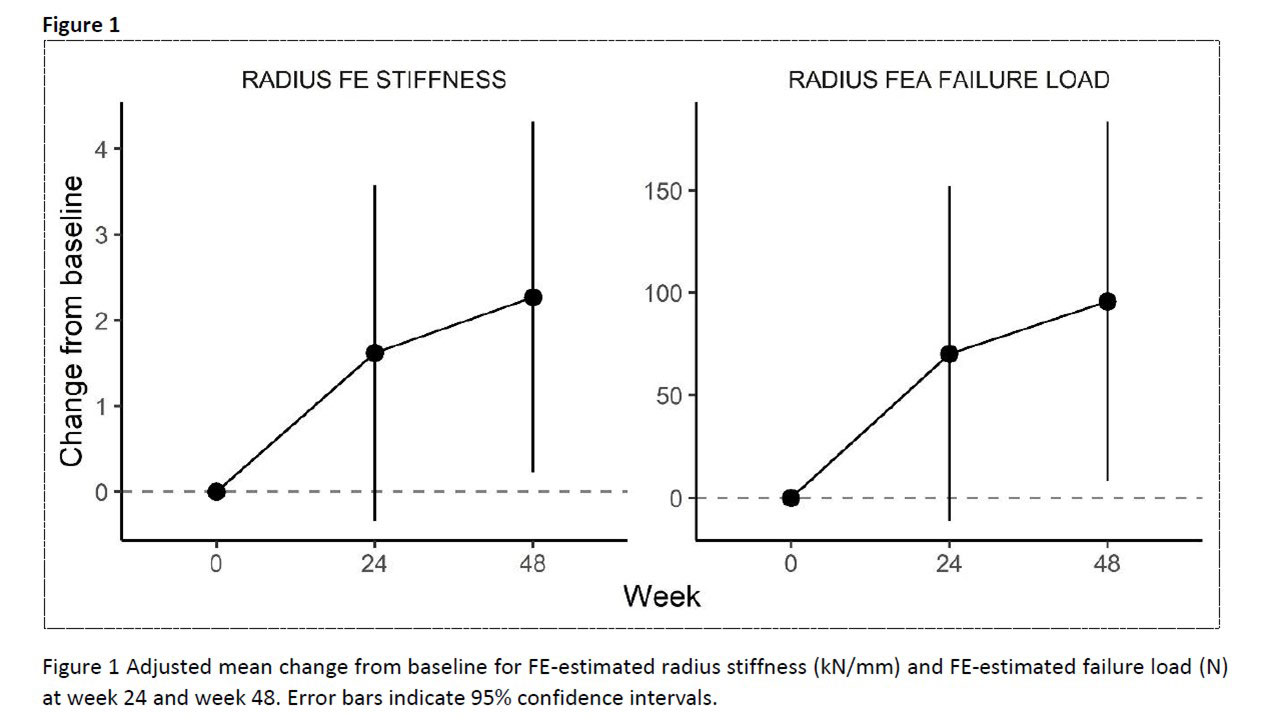
Results: Thirty- two RA patients were screened and 30 patients were included (age: 53.5 [SD 12.6] years; sex: f/m N 24/6; disease duration: 3 [IQR 2.0 – 8.0] years). HR-pQCT data for radial bone was available for 26 patients at week 48. The mean failure load increased from 1805 N at baseline to 1876 N (wk 24) and to 1882 N at week 48. Observed mean change from baseline is shown in Figure-1. Corresponding adjusted mean changes (95%CI) from baseline were 70.28 (-11.06 to 151.61) at wk 24 and 95.85 (8.49 to 183.21) at wk 48. A similar improvement was also observed in radial stiffness with statistical significance at wk 48 (Table-1). Of all changes observed in HR-pQCT measurements between baseline and week 48, a decrease in cortical porosity was associated with a larger improvement in failure load with a mean increase in change of 154.5 N (95%CI 38.1 to 270.8) for 0.01 decrease of change in cortical porosity, and also a larger improvement in stiffness with a mean increase in the change of 3.2 kN/mm (95%CI 0.9 to 5.6).
Conclusion: This is, to our knowledge, the first study showing an improvement of bone biomechanical properties in RA with tsDMARD treatment. BARI significantly improved failure load and stiffness of bone. Structurally cortical porosity was reduced by BARI. These effects occur relatively rapid after treatment initiation. Hence, JAK inhibition by baricitinib does not only inhibit inflammation and retards bone erosion in RA but also significantly improves the functional properties of bone with increased resistance to mechanical load.
1. McInnes, I.B. and G. Schett, N Engl J Med, 2011. 365(23): p. 2205_19.
2. Stemmler, F., et al., Ann Rheum Dis, 2018. 77(7): p. 973-980.
3. Pistoia, W., et al., Bone, 2002. 30(6): p. 842-8.
4. Adam, S., et al., Sci Transl Med, 2020. 12(530).
To cite this abstract in AMA style:
Kemenes S, Tascilar K, Simon D, Bayat S, Kroenke G, Valor Mendez L, Hartmann F, Schuster L, Liphardt A, Schett G, Kleyer A. Baricitinib Improves Bone Biomechanical Properties in Rheumatoid Arthritis (RA) – Results of a Prospective Interventional Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/baricitinib-improves-bone-biomechanical-properties-in-rheumatoid-arthritis-ra-results-of-a-prospective-interventional-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baricitinib-improves-bone-biomechanical-properties-in-rheumatoid-arthritis-ra-results-of-a-prospective-interventional-study/