Session Information
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Onset of autoimmune connective tissue disease (CTD) after COVID-19 infection has been reported and complement activation has been implicated. We examined common autoimmune rheumatic disease symptoms, serologies, and complement activation arising after COVID-19 infection.
Methods: We conducted a cross-sectional prospective study of patients who tested positive for COVID-19 from 3/1/20-9/1/21 at a large multihospital system. Eligible patients, ≥18 years old and ≥3 months post-COVID-19 infection, received the CTD Screening Questionnaire (CSQ; Karlson EW, 1995) and the Brief Pain Inventory (BPI) by electronic or physical mail. Subjects who returned completed questionnaires, had evidence of new CTD symptoms on CSQ, and had no prior CTD diagnosis (CSQ+) were invited for a physician exam and blood draw to test for autoimmune serologies and cell-bound complement activation products (EC4d, BC4d, and PC4d, together CB-CAPs). The multi-analyte assay panel (MAP) was determined using a two-tier algorithm. Targeting equal numbers of subjects who screened positive and negative on the CSQ, we invited age- and sex-matched subjects who had COVID-19, negative CSQs, and no prior CTD diagnosis (CSQ-) for blood collection. We tested for associations between CSQ positivity after COVID-19 and autoantibodies and CB-CAPs by Fisher exact test.
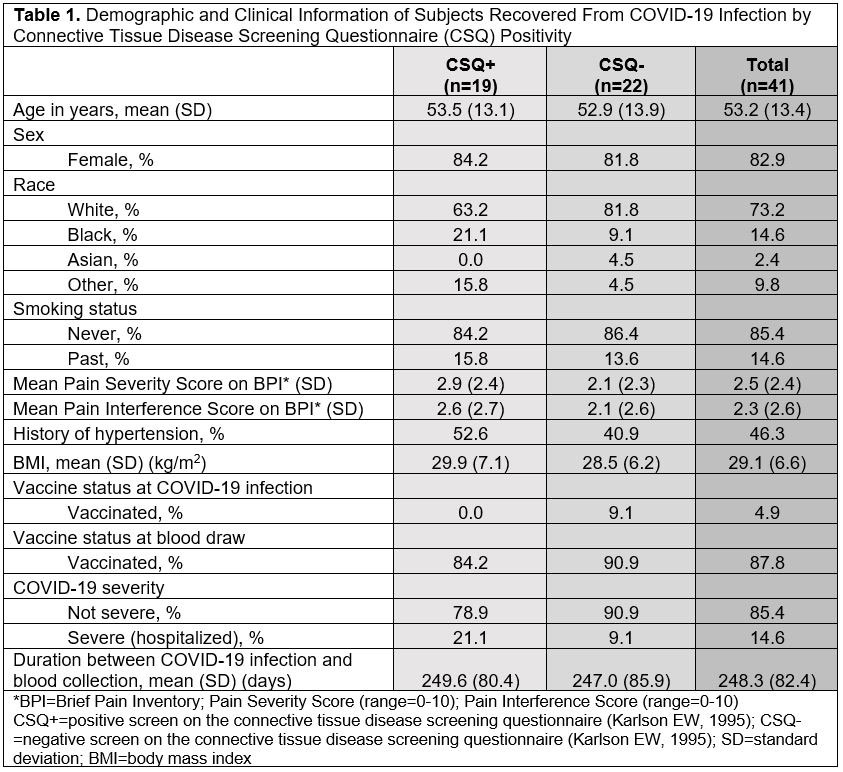
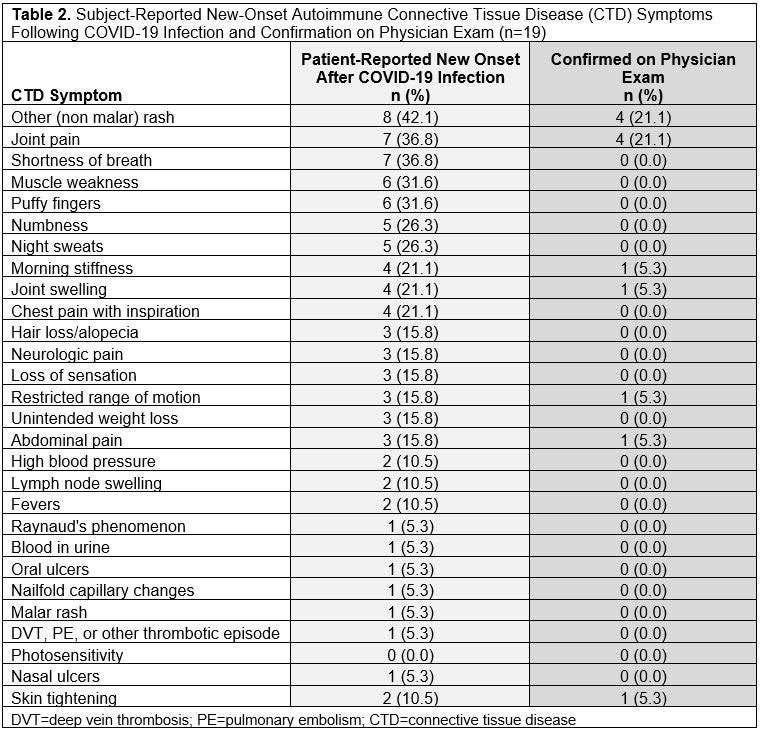
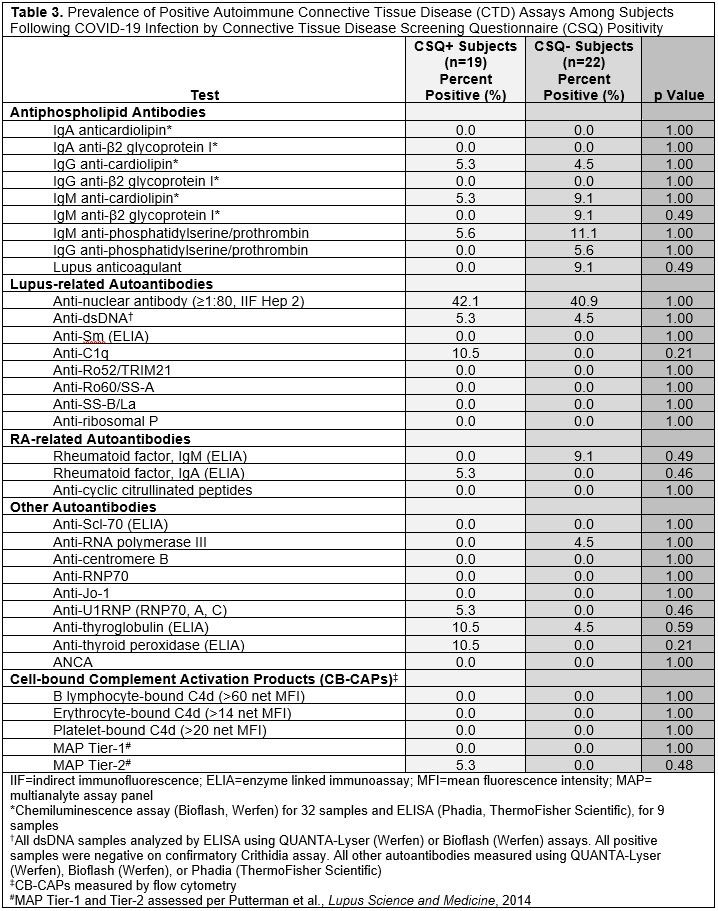
Results: We invited 101 eligible CSQ+ subjects and 122 CSQ- subjects to participate: 19 CSQ+ and 22 matched CSQ- subjects were enrolled. Age and sex were well-matched between the two groups (overall mean age 53.2 [SD 13.4], 82.9% female, 73.2% white). At time of infection, 4.9% of subjects were vaccinated and 14.6% were hospitalized (Table 1). Mean pain severity and interference scores were comparable among the CSQ+ and CSQ-. Among CSQ+ subjects, 11 subjects (57.9%) screened positive for symptoms of systemic lupus erythematosus (SLE), 8 (42.1%) screened positive for symptoms of rheumatoid arthritis (RA), and 2 (10.5%) screened positive for symptoms of scleroderma (SSc). Joint pain (36.8%), shortness of breath (36.8%), and other (non malar) rash (42.1%) were the most common subject-reported new onset CTD symptoms (Table 2). On exam, 4 cases of new joint pain and 4 cases of new non malar rash were found. There was an overall low prevalence of CTD autoantibodies and no positive CB-CAPs. No significant associations were identified between new CTD symptoms by CSQ and autoantibodies, CB-CAPs, or MAP (Table 3).
Conclusion: In this comparison of subjects following COVID-19 infection with or without self-reported new CTD symptoms, we found overall low prevalence of CTD symptoms on exam and CTD-related autoantibodies, and no associations between new CTD symptoms or physical exam findings and CTD autoantibodies, CB-CAPs, or MAP positivity. To our knowledge, this is the first study of CB-CAPs, a novel complement-based biomarker for SLE, in the context of COVID-19, which is thought to activate complement. These findings suggest that CSQ symptom positivity ≥3 months post-COVID-19 infection may not be associated with formation of CB-CAPs or CTD-associated autoantibodies.
To cite this abstract in AMA style:
Oakes E, Ellrodt J, Alexander R, Conklin J, Choi M, Guan H, Tedeschi S, Sparks J, Case S, Hsu T, Solomon D, Jonsson A, Costenbader K. Autoimmune Serologies, Cell-Bound Complement Activation Products, and Autoimmune Rheumatic Disease Symptoms After COVID-19 Infection [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/autoimmune-serologies-cell-bound-complement-activation-products-and-autoimmune-rheumatic-disease-symptoms-after-covid-19-infection/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/autoimmune-serologies-cell-bound-complement-activation-products-and-autoimmune-rheumatic-disease-symptoms-after-covid-19-infection/