Session Information
Date: Saturday, November 12, 2022
Title: SLE – Diagnosis, Manifestations, and Outcomes Poster I: Diagnosis
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Autoantibodies are a hallmark of lupus nephritis (LN). While there is known heterogeneity in autoantibody expression among LN patients, the association of autoantibodies with LN subtypes and the implications of longitudinal changes in LN are not entirely understood. In this study, we quantified circulating autoantibodies in the Accelerating Medicines Partnership (AMP) LN longitudinal cohort to identify novel serum biomarkers of LN classification and treatment response and to provide insights into the pathogenesis of LN.
Methods: SLE patients meeting ACR or SLICC criteria (n=268) indicated for kidney biopsy by a urine protein/creatinine (UPCR) >0.5 were recruited for this study as part of the AMP. Kidney biopsies were evaluated by a renal pathologist according to ISN/RPS classification, and serum samples were collected at the time of diagnostic kidney biopsy and 3-, 6-, and 12-months post-biopsy. Serum autoantibodies against dsDNA, chromatin, ribosomal-P, Ro, La, Sm, SmRNP, RNP, Jo-1, Scl-70, and centromere-B were measured using the BioPlex 2200® ANA kit (Bio-Rad Technologies, Hercules, CA), while anti-C1q positivity was determined by ELISA (QUANTA Lite®, Werfen, Bedford, MA). Clinical response was determined at 12 months using the Abatacept and Cyclophosphamide Combination Efficacy and Safety Study definitions for patients with ISN/RPS class III, IV, V, or a combination thereof and a baseline UPCR ratio >1.0.
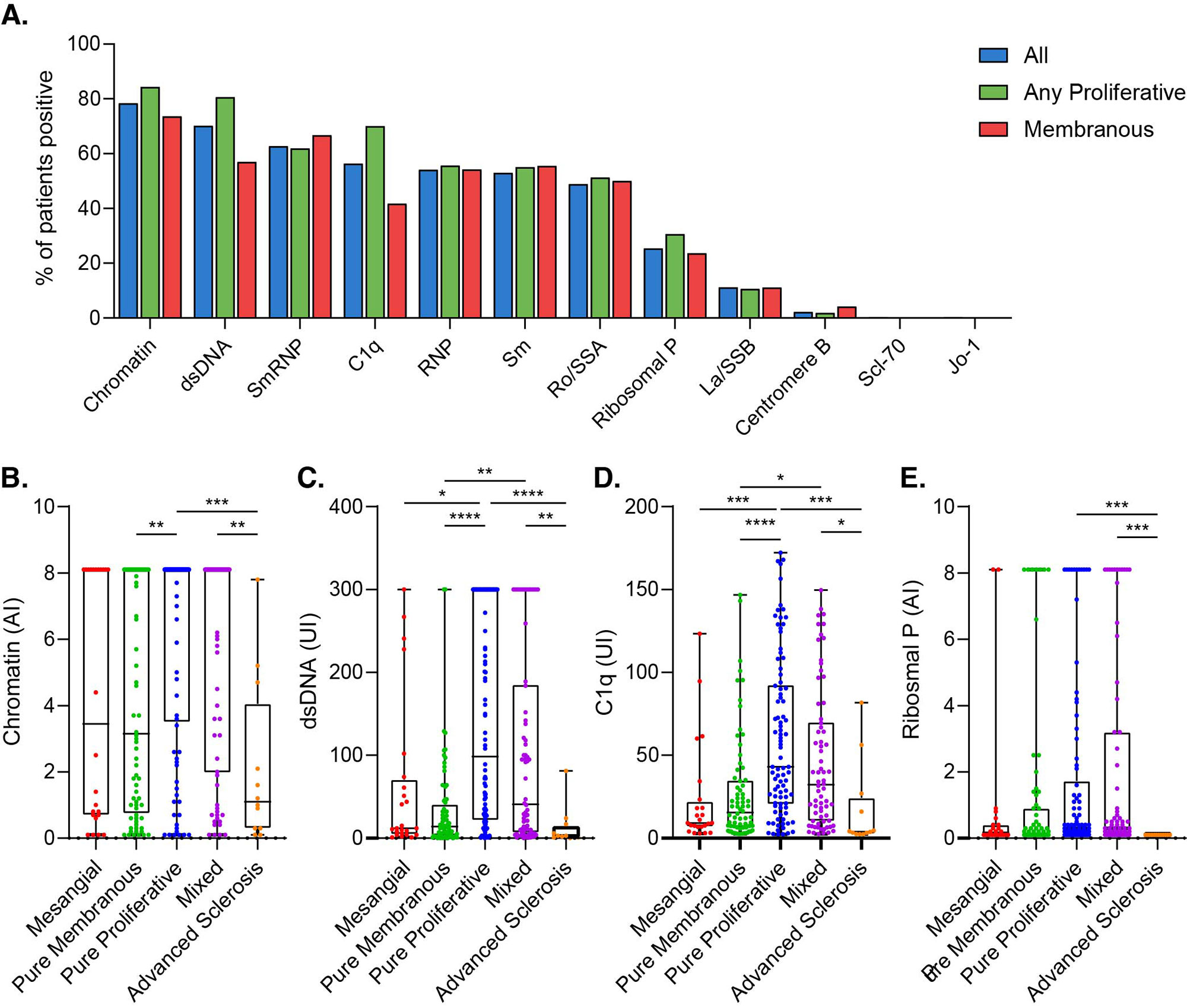
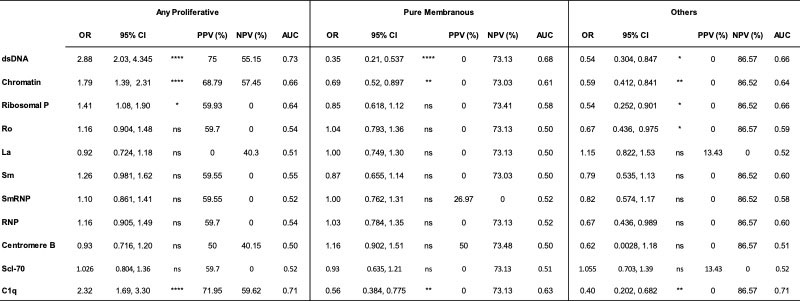
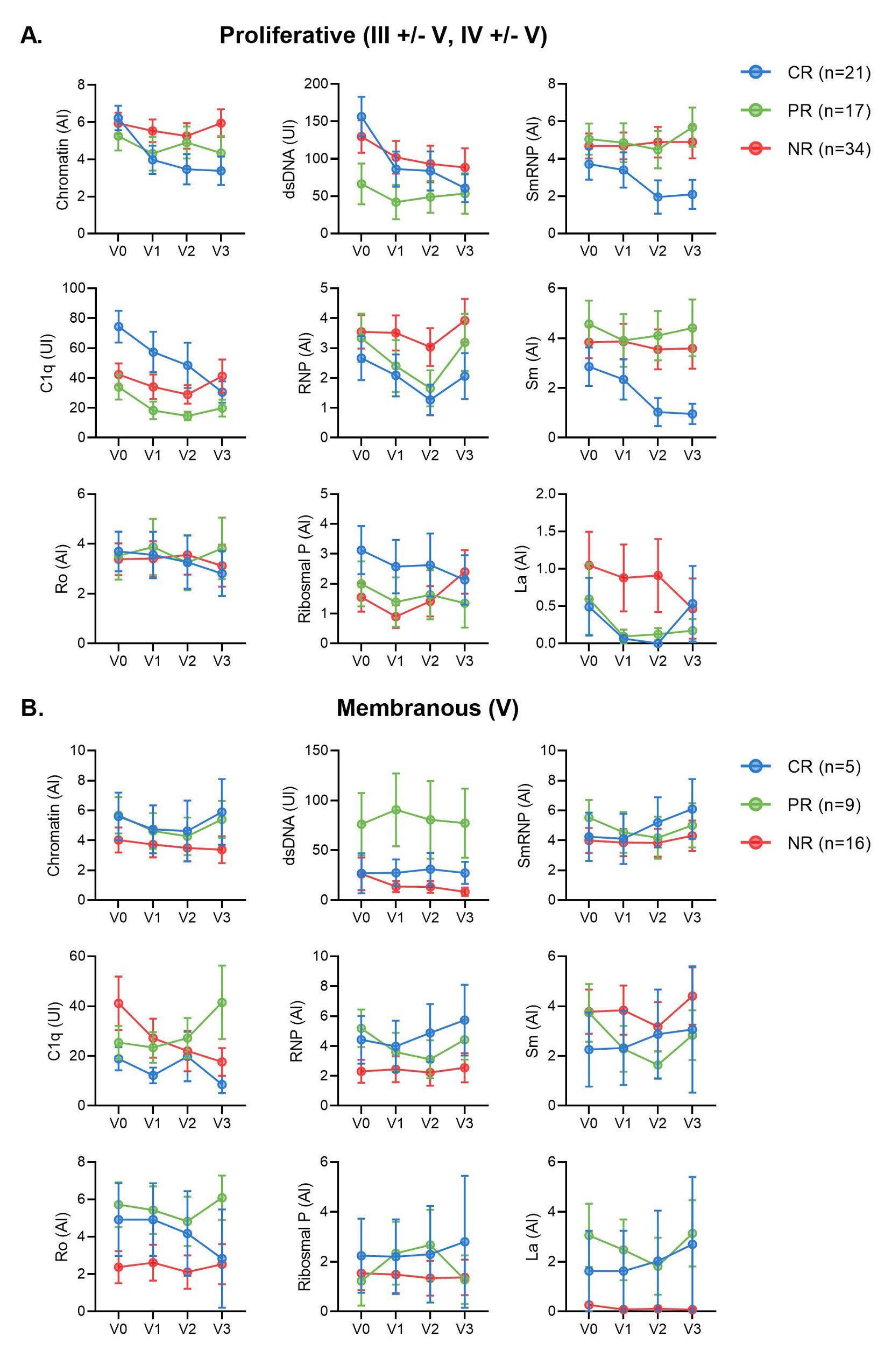
Results: Most LN patients exhibited autoantibodies against chromatin (78%) dsDNA (70%), SmRNP (63%), C1q (56%), RNP (54%), and Sm (52%) (Figure 1A). Patients with proliferative LN (class III, IV, III+V, or IV+V) had higher positivity rates of several autoantibodies, including those against dsDNA, chromatin, and C1q, compared to patients with membranous LN (class V) (Figure 1A). Similarly, patients with pure proliferative (class III or IV) and mixed (class III+V or IV+V) LN had significantly higher titers of these autoantibodies compared to those with mesangial (class I or II), membranous, and advanced sclerosis (class VI) LN (Figure 1B-D). Furthermore, increased titers of these autoantibodies were associated with higher odds of having proliferative LN (Table). Proliferative LN patients with a complete treatment response exhibited a significant decline in several autoantibodies including anti-dsDNA, C1q, chromatin, Smith, and ribosomal P (Figure 2). Autoantibody levels remained relatively stable in partial- and non-responder proliferative LN patients, as well as in patients with membranous LN.
Conclusion: LN patients exhibit heterogeneous autoantibody profiles associated with ISN/RPS classification. Specifically, levels of autoantibodies against dsDNA, C1q, chromatin, and ribosomal P may serve as noninvasive biomarkers of proliferative LN. In patients with proliferative but not membranous LN, a decline in the titers of several autoantibodies, including many not routinely measured over time, such as anti-Sm, was associated with treatment response, suggesting a possible role in LN pathogenesis. In addition, these autoantibodies may serve as early biomarkers of treatment response.
To cite this abstract in AMA style:
Fava A, Guthridge C, Kheir J, Wagner C, Petri M, Buyon J, Diamond B, (AMP) RA/SLE t, Guthridge J, James J. Autoantibody Trajectories Associate with Classification and Treatment Response in Lupus Nephritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/autoantibody-trajectories-associate-with-classification-and-treatment-response-in-lupus-nephritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/autoantibody-trajectories-associate-with-classification-and-treatment-response-in-lupus-nephritis/