Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Immune checkpoint inhibitors (ICI) are a novel cancer therapeutic that have had a dramatic impact on treating advanced malignancies by enhancing the anti-tumor T cell response. However, the ongoing activation of T cells also cause autoimmune side effects, termed immune-related adverse events (irAE). Given that some irAE draw comparisons to traditional autoimmune diseases, autoantibodies have been proposed as potential biomarkers to predict the occurrence of irAE.
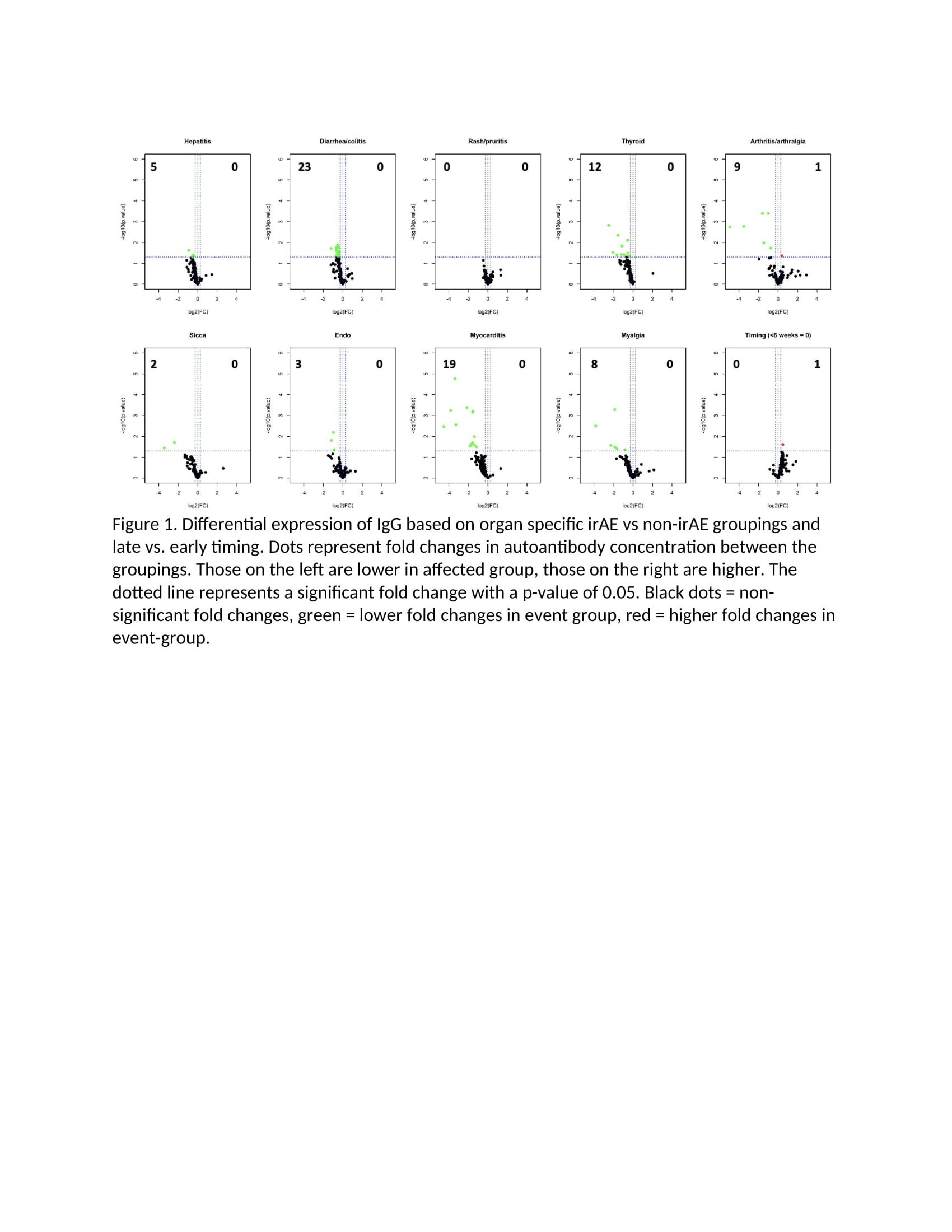
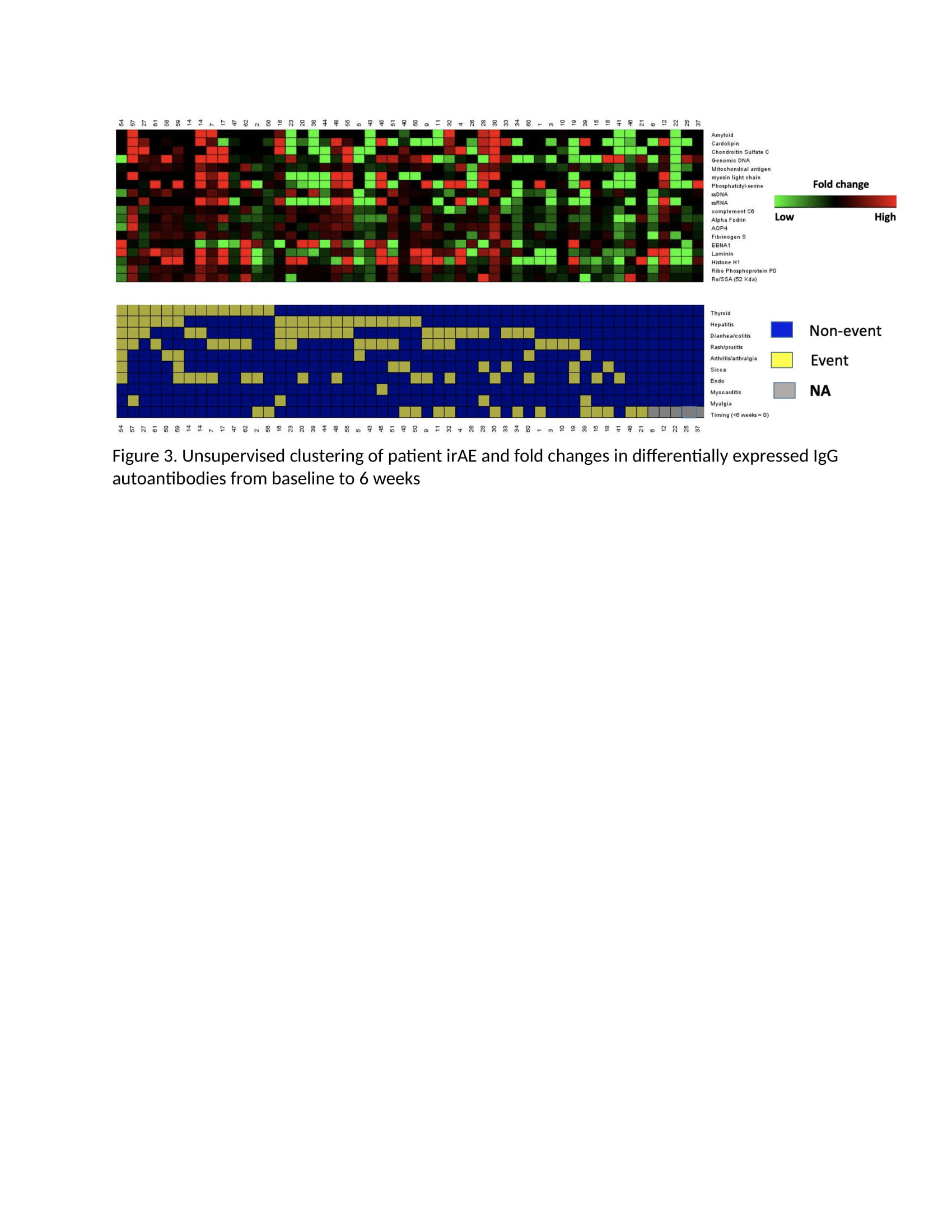
Methods: This study utilized clinical data and plasma collected from 60 patients enrolled in prospective phase II oncologic clinical trial of two doses of combination ICI (anti-CTLA4/anti-PD1) followed by anti-PD1 monotherapy for the treatment of advanced melanoma (PI Postow). Plasma was collected, frozen and stored at baseline and week 6 after treatment initiation. Samples were later thawed and incubated with a 120-autoantigen microarray to identify IgM and IgG autoantibodies commonly associated with autoimmune diseases. Average signal intensities of autoantibodies both at baseline and fold changes between the baseline and week 6 timepoint were compared among patients with vs. without an organ-specific irAE. Student’s t test was used to determine if there was a significant difference between average signal intensities for each antibody across toxicity groups, and log2 fold changes (FC) and p-values were recorded for each antibody. Statistically significant changes in differential expression are noted in the figures as green (negative FC) and red (positive FC) dots. Unsupervised clustering was also performed.
Results: The 60 patients had a mean age (SD) of 60.4 (SD 13.3), 63% male. Ninety-two percent experienced at least one irAE and 63% had their first irAE before 6 weeks. Patients who experienced an organ-specific irAE (e.g. colitis) tended to have fewer autoantibodies at baseline than patients who did not experience that irAE (Figure 1). Additionally, patients who experienced any irAE before 6 weeks had fewer autoantibodies at baseline than those with irAE only after 6 weeks (Figure 1). There were essentially no baseline autoantibodies whose presence predicted an organ-specific irAE. Unsupervised clustering suggested that patients with irAE had a greater fold change in IgM (Figure 2) and IgG (Figure 3) from baseline to 6 weeks than those without irAE, and the same was true for patients who experienced an irAE before compared to after 6 weeks.
Conclusion: A low level of baseline autoantibodies and an increase in autoantibodies after ICI treatment were associated with organ specific irAE. This finding may reflect a skewing toward cellular immunity in these patients prone to irAE rather than a humoral dispensation.
To cite this abstract in AMA style:
Ghosh N, Zhu C, Chan K, Postow M, Bass A. Autoantibody Microarray Signal Intensities May Predict the Occurrence of Immune-related Adverse Events [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/autoantibody-microarray-signal-intensities-may-predict-the-occurrence-of-immune-related-adverse-events/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/autoantibody-microarray-signal-intensities-may-predict-the-occurrence-of-immune-related-adverse-events/