Session Information
Date: Monday, November 9, 2020
Title: RA – Diagnosis, Manifestations, & Outcomes Poster IV: Lifespan of a Disease
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Disease burden and subjective symptoms of rheumatoid arthritis (RA) remain even after achieving clinical remission or low disease activity. Impairments to work and societal/daily activity are relevant burdens for patients with RA. However, detailed analyses are rarely performed. For exploring a better strategy aiming at less burden beyond Treat-to-Target strategy, it is essential to evaluate details of subjective symptoms collected as patient-reported-outcomes (PROs) and their relationship to work productivity and activity impairment (WPAI). The purpose of this study was to explore patient-reported outcomes (PROs) related to WPAI in patients with RA who achieved clinical remission.
Methods: The Institute of Rheumatology Rheumatoid Arthritis (IORRA) database is an established cohort database with RA in Tokyo Women’s Medical University since 2000. RA patients ≥18 years who met the 1987 American College of Rheumatology classification criteria for RA and SDAI remission criteria (SDAI<3.3) within the IORRA dataset collected in October 2017 were used for this analysis. Pain-VAS [0–100 mm], patients’ global assessment VAS (Pt-GA) [0–100 mm], Healthcare Assessment Questionnaire-Disability Index (Japanese version; J-HAQ-DI), and duration of morning joint stiffness (MJS) were evaluated. To measure impact on work productivity during the past 7 days, the WPAI-RA instrument (absenteeism, presenteeism, work productivity loss, and daily activity impairment) was used. Spearman’s rank correlation coefficient with 95% confidence interval (95% CI) was calculated to evaluate correlation between each WPAI item and each PRO. To evaluate the degree of contribution of each PRO to WPAI, an ANOVA model was constructed. The contribution of each PRO was calculated based on the sum squares of each PRO divided by total sum squares.
Results: Mean age of the 2,614 patients was 62.4 years; 85.1% were female; and mean DAS28-ESR and SDAI was 2.0 and 1.3, respectively. Mean scores of WPAI were 1.1% for absenteeism (percent work time missed due to RA), 6.5% for presenteeism (percent impairment while working due to RA), 7.4% for work impairment, and 10.2% for activity impairment. Mean pain VAS and Pt-GA were 7.1 and 7.5, respectively, and mean J-HAQ-DI score was 0.3. MJS was reported in 17.6% of patients with a mean duration of 45.3 minutes. Pain VAS showed the strongest positive correlation with each score of WPAI except for absenteeism (Spearman’s rank correlation coefficient [95% CI] 0.52 [0.47, 0.56] for presenteeism, 0.50 [0.45, 0.55] for work impairment, and 0.60 [0.57, 0.63] for activity impairment) (Table 1). MJS contributed the most to absenteeism (18.0%) while pain-VAS contributed the most to presenteeism (57.4%), work productivity loss (51.1%), and daily activity impairment (53.7%) (Table 2). J-HAQ-DI was the second most contributing factor to all scores of WPAI (6.0% for absenteeism, 17.4% for presenteeism, 16.3% for work productivity loss, and 26.0% for daily activity impairment).
Conclusion: Pain-VAS and J-HAQ-DI highly contributed to WPAI. This study indicates that improvement in these PROs may lead to less burden of RA patients in clinical remission.
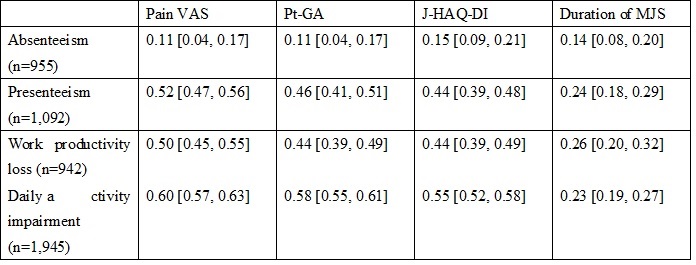 Correlation coefficient between WPAI and each PRO. Values are Spearman’s rank correlation coefficient [95% confidence interval]. WPAI, Work Productivity and Activity Impairment; PRO, patient-reported outcomes; VAS, visual analogue scale; J-HAQ-DI, Japanese version of Healthcare Assessment Questionnaire-Disability Index; MJS, morning joint stiffness
Correlation coefficient between WPAI and each PRO. Values are Spearman’s rank correlation coefficient [95% confidence interval]. WPAI, Work Productivity and Activity Impairment; PRO, patient-reported outcomes; VAS, visual analogue scale; J-HAQ-DI, Japanese version of Healthcare Assessment Questionnaire-Disability Index; MJS, morning joint stiffness
 Contribution to variance in WPAI score. WPAI, Work Productivity and Activity Impairment; RA, Rheumatoid arthritis; BMI, Body Mass Index; TJC, Tender joint count; SJC, Swollen joint count; CRP,C-reactive protein; ESR, Erythrocyte sedimentation rate; Ph-GA, Physician’s global assessment; CCI, Charlson Comorbidity Index; PRO, patient-reported outcomes; VAS, visual analogue scale; Pt-GA, Patient’s global assessment; J-HAQ-DI, Japanese version of Healthcare Assessment Questionnaire-Disability Index; MJS, morning joint stiffness
Contribution to variance in WPAI score. WPAI, Work Productivity and Activity Impairment; RA, Rheumatoid arthritis; BMI, Body Mass Index; TJC, Tender joint count; SJC, Swollen joint count; CRP,C-reactive protein; ESR, Erythrocyte sedimentation rate; Ph-GA, Physician’s global assessment; CCI, Charlson Comorbidity Index; PRO, patient-reported outcomes; VAS, visual analogue scale; Pt-GA, Patient’s global assessment; J-HAQ-DI, Japanese version of Healthcare Assessment Questionnaire-Disability Index; MJS, morning joint stiffness
To cite this abstract in AMA style:
Sakai R, Tanaka E, Inoue E, Sato M, Tanaka M, Ikari K, Taniguchi A, Yamanaka H, Harigai M. Associations Between Patient Reported Outcomes and Impairments of Work and Activity in Patients with Rheumatoid Arthritis Who Achieved Clinical Remission; Retrospective Analysis Using the IORRA Database [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/associations-between-patient-reported-outcomes-and-impairments-of-work-and-activity-in-patients-with-rheumatoid-arthritis-who-achieved-clinical-remission-retrospective-analysis-using-the-iorra-databa/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/associations-between-patient-reported-outcomes-and-impairments-of-work-and-activity-in-patients-with-rheumatoid-arthritis-who-achieved-clinical-remission-retrospective-analysis-using-the-iorra-databa/
