Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Patients with rheumatoid arthritis (RA) are at increased risk of hospitalizations and mortality due to RA itself, associated comorbidities, and treatment-related complications. The purpose of this real-world study was to investigate the association between RA disease activity, physical function, comorbidity, and anti-rheumatic medications and the risk of all-cause mortality.
Methods: RA patients enrolled in the Ontario Best Practices Research Initiative (OBRI) between 1st of June 2008 and 1st of Jan 2023 were included. Patients were eligible if they had clinical disease activity index (CDAI) and health assessment questionnaire disability index (HAQ-DI) scores at cohort entry and ≥ 6 months of follow-up. Patients also had to be be on at least one anti-rheumatic medication. Multiple imputation (Imputation Chained Equation, N=20) was used to deal with missing data. We conducted multivariable Cox regression analyses to estimate the hazard of death, controlling for sociodemographic, clinical, medication and comorbidity factors. All variables included in the regression models were time-dependent except BMI, Gender, Positive RF, and Education which were only measured at cohort entry.
Results: A total of 3384 patients were included. 78.4% were female and mean (SD) age and disease duration were 57.9 (12.9) years and 8.2 (9.8) years, respectively. The mean (SD) CDAI was 20.2 (13.6) and HAQ-DI was 1.1 (0.8). Over a median 77.7 months follow-up, 218 deaths (6.4%) were recorded.
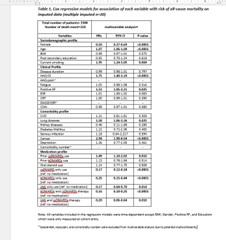
Table 1 shows the results for multivariable analysis. Use of csDMARD (HRs: 0.17; 95%CI: 0.12-0.24), bDMARD mono (HRs: 0.25; 95%CI: 0.15-0.44), tsDMARD mono (HRs: 0.17; 95%CI: 0.04-0.70), bDMARD/csDMARD (HRs: 0.16; 95%CI: 0.10-0.25), and tsDMARD/csDMARDs (HRs: 0.20; 95%CI: 0.06-0.64) showed a significantly negative association with all-cause mortality.
With respect to clinical profile, only higher HAQ-DI (HRs: 1.75; 95%CI: 1.40-2.19) and positive RF (HRs: 1.53; 95%CI: 1.05-2.21) showed a significant association with risk of death. Lung disease (HRs: 1.58; 95%CI: 1.06-2.36), cancer (HRs: 2.94; 95%CI: 1.90-4.54), current smoking (HRs: 1.95; 95%CI: 1.24-3.05), use of csDMARDs before enrolment (HRs: 1.49; 95%CI: 1.10-2.02) were also significantly associated with risk of death.
Conclusion: In this real-world study, we found that higher HAQ-DI, lung disease and cancers were associated with all-cause mortality in RA but the use of csDMARD, bDMARD and tsDMARDs were negatively associated with all-cause mortality in patients with RA.
To cite this abstract in AMA style:
Movahedi M, Cesta A, Li X, Kuriya B, Aydin S, Keystone E, Pope J, Bombardier C. Associations Between Disease Activity, Physical Function and Anti-rheumatic Medications with All-cause Mortality in Rheumatoid Arthritis (RA): Data from a Canadian RA Registry [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/associations-between-disease-activity-physical-function-and-anti-rheumatic-medications-with-all-cause-mortality-in-rheumatoid-arthritis-ra-data-from-a-canadian-ra-registry/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/associations-between-disease-activity-physical-function-and-anti-rheumatic-medications-with-all-cause-mortality-in-rheumatoid-arthritis-ra-data-from-a-canadian-ra-registry/