Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: People with autoimmune rheumatic diseases (AIRD) are at higher risk for serious complications and poor outcomes from COVID-19 infection. Data is lacking on outcomes related to the 2023-2024 updated COVID-19 vaccine in this population. We aimed to assess the association between vaccination status and COVID-19-related hospital admissions.
Methods: We conducted a retrospective cohort analysis using electronic health record (EHR) data from the National Clinical Cohort Collaborative (N3C) COVID-19 Enclave among people with AIRD and incident positive COVID-19 testing (i.e., positive SARS-CoV-2 polymerase chain reaction (PCR), antigen, or antibody test or COVID-19 diagnostic code) between December 2020 and December 2023. Eligible patients were adults age ≥18 years with ≥2 International Classification of Diseases (ICD)-10 codes for AIRD occurring at least 30-days apart. We defined time-varying vaccination status as: unvaccinated (i.e., no vaccine record on file), initial series completed (i.e., received two mRNA vaccine doses or 1 Janssen dose of the COVID-19 vaccine), boosted (i.e., received initial vaccination series and a booster or bivalent dose), or updated vaccinated (with 1+ updated 2023–2024 COVID-19 vaccine). We defined the primary outcome as presence of an inpatient hospitalization occurring during the period 3 days before and up to 14 days after positive SARS-CoV-2 testing, and allowed for repeat hospitalizations. We conducted a multivariable logistic regression model and adjusted for repeating measures and participating site. Covariates included sex, age, race and ethnicity, AIRD category, AIRD-related medication use, tobacco use, presence of hypertension, obesity, modified Charlson comorbidity index (excluding AIRD), and social vulnerability index (SVI).
Results: Our cohort included 54,848 people with AIRD, including 41,383 (75%) females, with median (IQR) age 62 (50-73) and 39,766 (73%) White non-Hispanic race (Table 1). Of these, 5,775 (11%) had received an updated 2023-2024 COVID-19 vaccine. After adjusting for relevant covariates, people with AIRD who had received a booster or an updated vaccine dose had 71% (adjusted odds ratio [AOR]=0.29, 95% confidence interval [CI] 0.27-0.31) and 73% (AOR=0.27, 95% CI 0.25-0.30) lower odds of COVID-19-related hospitalization compared to unvaccinated people, with similar odds comparing to those with initial series alone (Figure 1). In addition, male sex, older age, Black non-Hispanic race, having systemic lupus erythematosus, exposure to CD20 inhibitors, immunosuppressives, or glucocorticoids, current tobacco use, having hypertension, more comorbidities, and higher SVI were associated with an increased odds of COVID-19-related hospitalization.
Conclusion: After accounting for important covariates, we observed a substantially decreased independent odds of COVID-related hospitalization among people with AIRD that received a booster or updated 2023-2024 COVID-19 vaccine compared to initial series alone or unvaccinated statuses. Updated vaccinations appear to continue to provide significant protection for this vulnerable population and should be supported by providers, policy makers, and patient advocacy groups.
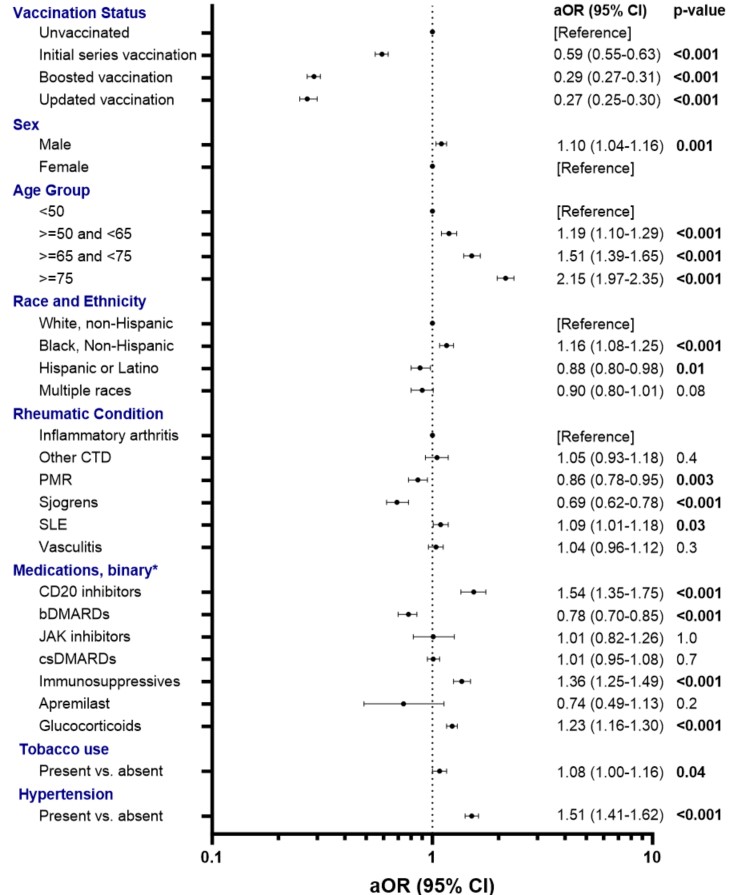 Inflammatory arthritis includes rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, enteropathic arthritis, and reactive arthritis. Other CTD includes polymyositis, dermatomyositis, and systemic sclerosis. Vasculitis includes Behcet’s disease, ANCA-vasculitis, giant cell arteritis, and polyarteritis nodosa. CCI, Charlson Comorbidity Index; CTD, connective tissue disease; JAK inhibitor, janus kinase inhibitor; PMR, polymyalgia rheumatic; SLE, systemic lupus erythematosus; SVI, social vulnerability index. P < 0.05 indicated in bold.
Inflammatory arthritis includes rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, enteropathic arthritis, and reactive arthritis. Other CTD includes polymyositis, dermatomyositis, and systemic sclerosis. Vasculitis includes Behcet’s disease, ANCA-vasculitis, giant cell arteritis, and polyarteritis nodosa. CCI, Charlson Comorbidity Index; CTD, connective tissue disease; JAK inhibitor, janus kinase inhibitor; PMR, polymyalgia rheumatic; SLE, systemic lupus erythematosus; SVI, social vulnerability index. P < 0.05 indicated in bold.
.jpg) Figure 1. Results of multivariable logistic regression model with odds ratio (aOR) and 95% confidence intervals (CI) adjusted for covariates associated with COVID-19 related hospitalization. OR and CI presented on a logarithmic scale. Model was adjusted for: vaccination status, sex, age, race and ethnicity, rheumatic condition, medication use, tobacco use, presence of hypertension, Charlson Comorbidity Index, and social vulnerability index.
Figure 1. Results of multivariable logistic regression model with odds ratio (aOR) and 95% confidence intervals (CI) adjusted for covariates associated with COVID-19 related hospitalization. OR and CI presented on a logarithmic scale. Model was adjusted for: vaccination status, sex, age, race and ethnicity, rheumatic condition, medication use, tobacco use, presence of hypertension, Charlson Comorbidity Index, and social vulnerability index.
*categories defined by presence vs. absence of medication. bDMARDs, biologic disease modifying anti-rheumatic drugs; csDMARDs, conventional synthetic disease modifying anti-rheumatic drugs; JAK inhibitor, janus kinase inhibitor; PMR, polymyalgia rheumatic; SLE, systemic lupus erythematosus.
To cite this abstract in AMA style:
Jackson L, anzalone j, Begum R, Rahman F, Singh N, Robinson L, Michaud K, Saag K, Singh J, Patel R, Danila M. Association between newer COVID-19 vaccines and COVID-19 related hospitalizations among people with autoimmune rheumatic diseases in the U.S. National COVID Cohort Collaborative (N3C) [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/association-between-newer-covid-19-vaccines-and-covid-19-related-hospitalizations-among-people-with-autoimmune-rheumatic-diseases-in-the-u-s-national-covid-cohort-collaborative-n3c/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/association-between-newer-covid-19-vaccines-and-covid-19-related-hospitalizations-among-people-with-autoimmune-rheumatic-diseases-in-the-u-s-national-covid-cohort-collaborative-n3c/
