Session Information
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Recently, CD45−CD31−Podoplanin (PDPN)+ synovial fibroblast-like cells, termed pre-inflammatory mesenchymal (PRIME) cells were found to be differentially expressed and circulate in the blood of rheumatoid arthritis (RA) patients before an arthritic flare (1). PRIME cells are hypothesized to be stimulated from an inflammatory trigger and recruited to the synovium from blood to facilitate flares, suggesting that their presence in circulating blood can be a biomarker for inflammatory arthritis. However, the presence of a PRIME cell population in rodent animal models of RA has yet to be determined. TNF-transgenic (TNF-Tg) mice are a model of RA in which the constitutive expression of pro-inflammatory cytokines characterizes a persistent inflammation during disease development. Identification of PRIME cells in rodent models, and specifically in the TNF-Tg mice, may be utilized to further elucidate the role of PRIME cells in inflammatory arthritis. Thus, we hypothesized that arthritic TNF-Tg mice express a population of circulating PRIME cells that are increased compared to wild-type (WT) controls.
Methods: Flow cytometry analysis of peripheral blood and bone marrow was performed on 6-9-month-old TNF-Tg and WT male littermates (n=3 mice/group). PRIME cells were determined as CD45-CD31-PDPN+ gated cells, and for each experimental sample, populations were compared with the percentage of gated cells and the total count of PRIME cells (Fig 1). Groups were analyzed using unpaired t-tests, and values are reported as mean ± standard deviation.
Results: At 6-9 months, both TNF-Tg and WT mice showed PRIME cell populations in circulating blood (2.28 ± 1.98% TNF-Tg vs 2.21 ± 3.52% WT; 31.33 ± 45.88 TNF-Tg vs 31.33 ± 53.41 WT) and bone marrow (7.70 ± 13.34% TNF-Tg vs 12.11 ± 20.98% WT; 25 ± 43.3 TNF-Tg vs 35.33 ± 61.20 WT). There was no significant difference in PRIME cell populations between TNF-Tg and WT mice.
Conclusion: These preliminary results suggest that PRIME cells can be detected in blood and bone marrow of both TNF-Tg mice with advanced arthritis and their WT littermates. However, by late stage arthritis, the PRIME cell population in TNF-Tg mice is not greater than that found in WT controls, and therefore ineffective as a biomarker of chronic disease in this model. Future studies looking at early disease and flare are warranted to further clarify the use of PRIME cells as biomarkers in TNF-induced arthritis.
1. Orange D.E. et al. N Engl J Med 383(3):218-228. 2020.
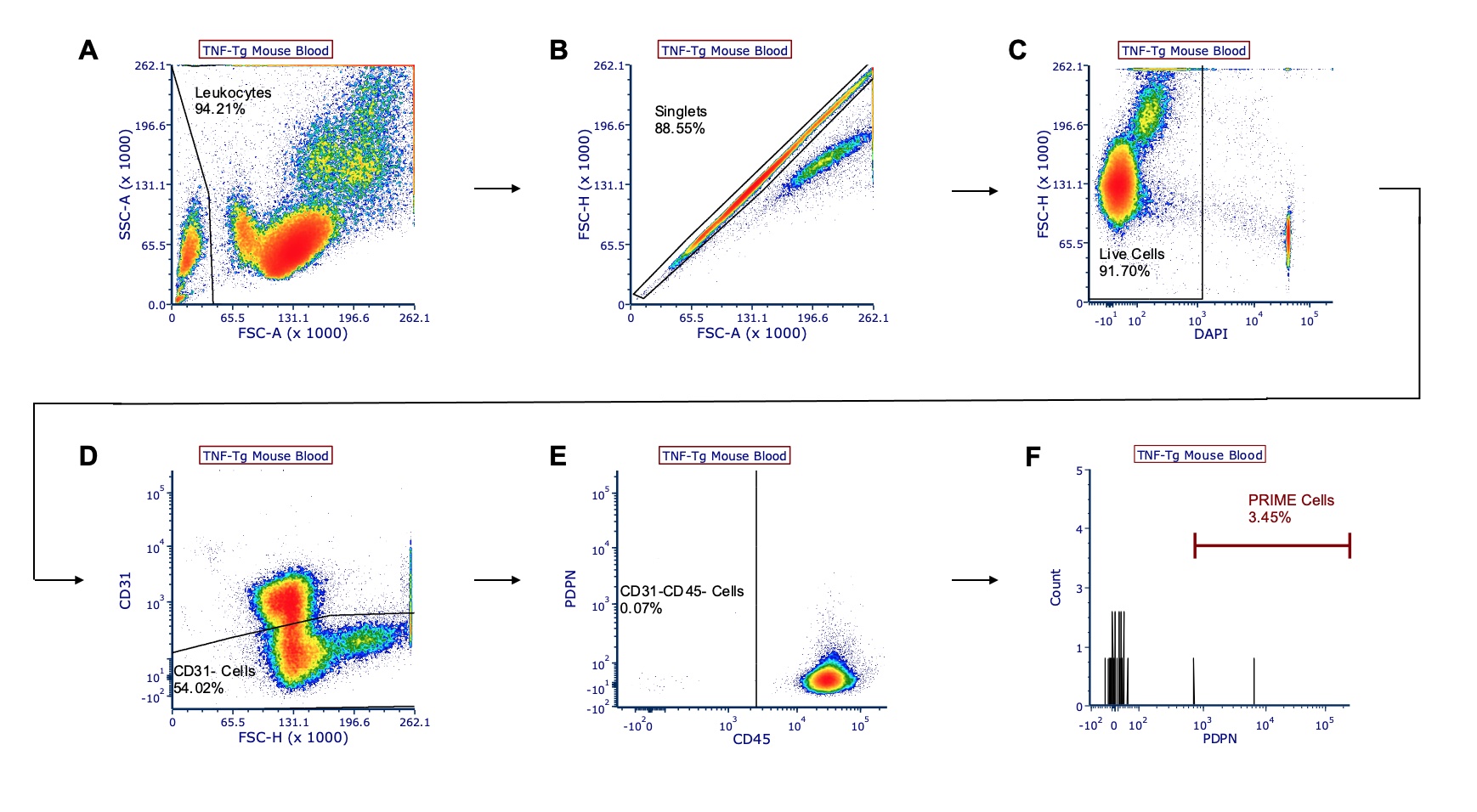 Figure 1. Gating strategy of representative TNF-Tg mouse blood for CD45-CD31-PDPN+ PRIME cells. TNF-Tg mouse peripheral blood (male; 6.5-months old) was harvested and stained with CD45, CD31, and podoplanin (PDPN) antibodies and DAPI viability stain. Live, single-cell leukocytes were gated (A-C), and PRIME cells were identified as CD31- (D), CD45- (E), and PDPN+ (F) cells. Each value represents the percentage of gated cells.
Figure 1. Gating strategy of representative TNF-Tg mouse blood for CD45-CD31-PDPN+ PRIME cells. TNF-Tg mouse peripheral blood (male; 6.5-months old) was harvested and stained with CD45, CD31, and podoplanin (PDPN) antibodies and DAPI viability stain. Live, single-cell leukocytes were gated (A-C), and PRIME cells were identified as CD31- (D), CD45- (E), and PDPN+ (F) cells. Each value represents the percentage of gated cells.
To cite this abstract in AMA style:
Chen K, Lin X, Xing L, Kenney H, Bell R, Schwarz E, Rahimi H. Assessment of Pre-Inflammatory Mesenchymal (PRIME) Cells as a Biomarker of Tumor Necrosis Factor-Induced Arthritis in Mice [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/assessment-of-pre-inflammatory-mesenchymal-prime-cells-as-a-biomarker-of-tumor-necrosis-factor-induced-arthritis-in-mice/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessment-of-pre-inflammatory-mesenchymal-prime-cells-as-a-biomarker-of-tumor-necrosis-factor-induced-arthritis-in-mice/
