Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Interstitial lung disease (ILD) is a common manifestation of idiopathic inflammatory myopathies (IIM) ranging up to 78% in IIM and is a key contributor to hospitalization, excess morbidity and mortality. In-vivo visualization evidence of ongoing tissue remodeling in IIM-ILD is scarce. In this study, we aimed to quantify and compare fibroblast activation in the lungs of IIM-patients and control subjects using ⁶⁸Ga-labelled inhibitor of Fibroblast-Activation-Protein based (⁶⁸Ga-DATA.SA.FAPI) positron emission tomography combined with computed tomography (FAPI PET/CT) imaging.
Methods: Patients with IIM recruited prospectively from the rheumatology outpatient clinic, and control subjects without rheumatic conditions or ILD recruited from the cardiology outpatient clinic underwent FAPI PET-CT imaging. Pulmonary FAPI accumulation was assessed by measuring the maximal standardized uptake (SUV) value (SUVmax) and mean SUV (SUVmean) over the whole lung (wl) using the liver as internal reference, respectively. Values of SUV were compared across IIM patients with and without ILD and controls using analysis of variance test and displayed as mean ± standard deviation (SD). Standard-of-care procedures such as high-resolution computed tomography (hr-CT) and pulmonary function testing (PFT) were performed in all IIM-patients at baseline and in patients with ILD at follow-up.
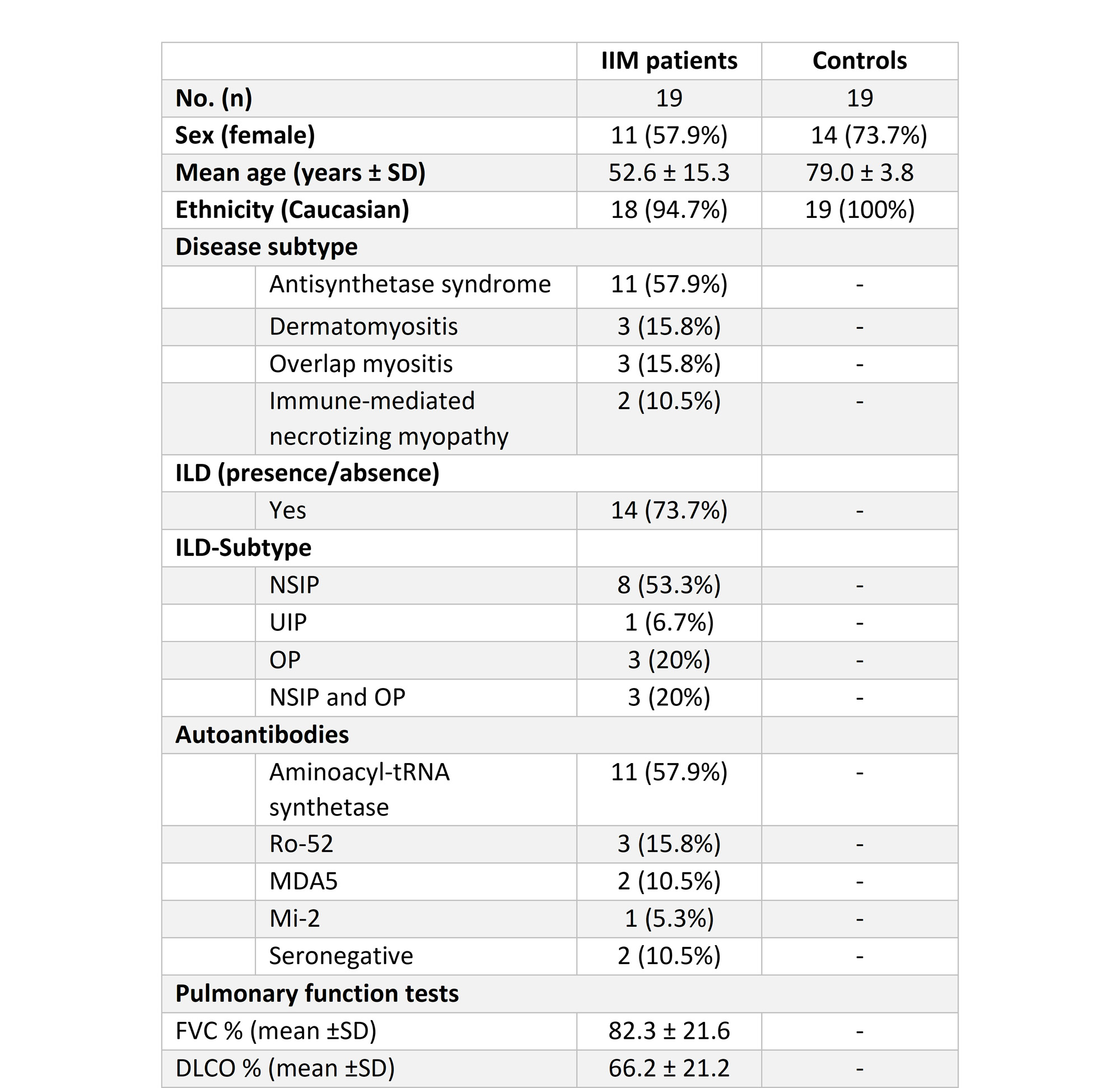
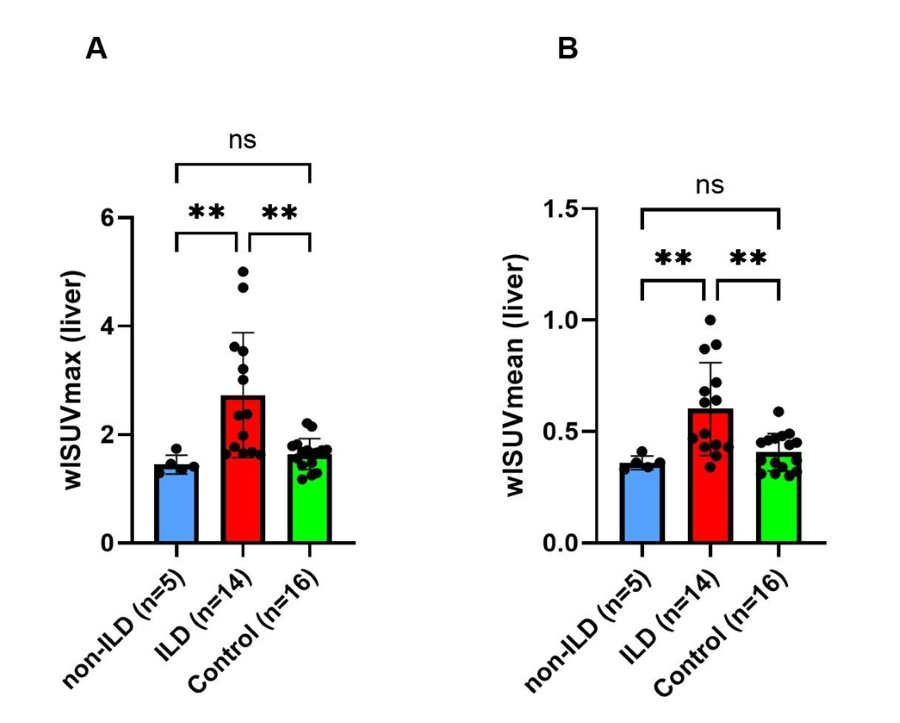
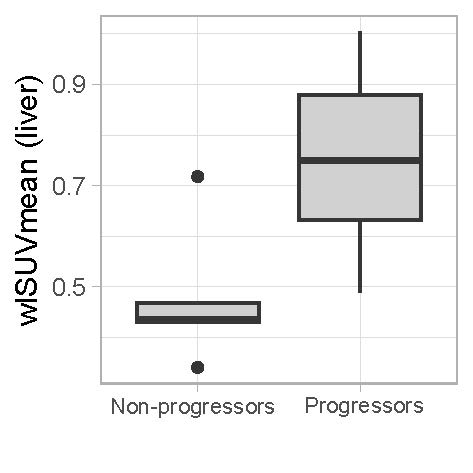
Results: The clinical characteristics of patients with IIM (14 patients with ILD confirmed by hr-CT and 5 non-ILD patients with primary muscular affection) and control subjects (n=19) are displayed in Table 1. Three (n=3) patients in the control group were excluded from analysis due to pulmonary disease or cardiac decompensation. In individuals with IIM-related ILD, whole-lung ⁶⁸Ga-DATA.SA.FAPI uptake assessed by wlSUVmax (Figure 1A) and wlSUVmean (Figure 1B) corrected for the liver was significantly increased as compared to both non-ILD IIM patients and the control group. No differences of wlSUVmax or wlSUVmean were observed between non-ILD IIM patients and the control group. FAPI uptake in the lungs correlated significantly with pulmonary function tests, severity of dyspnea and serum concentration of acute phase reactants at baseline. Moreover, IIM-patients with progressive ILD (defined as worsening in pulmonary function tests at 1-year follow up and/or need for intensification of immunosuppressive therapy) had significantly increased pulmonary FAPI uptake at baseline (Figure 2).
Conclusion: Our study demonstrates higher FAPI uptake in patients with IIM-ILD. Intensity of pulmonary FAPI accumulation was associated with progression of ILD. Thus, ⁶⁸ Ga-DATA.SA.FAPi PET/CT may serve as a useful non-invasive tool for risk stratification of lung disease in IIM.
To cite this abstract in AMA style:
Kastrati K, Nakuz T, Kulterer O, Blüml S, Bonelli M, Gessl I, Kiener H, Langsteger W, Mrak D, Prayer F, Prosch H, Simader E, Traub-Weidinger T, Aletaha D, Radner H, Hacker M, Mandl P. Assessment of Myositis-related Interstitial Lung Disease by ⁶⁸ Ga-DATA.SA.FAPi PET/CT [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/assessment-of-myositis-related-interstitial-lung-disease-by-%e2%81%b6%e2%81%b8-ga-data-sa-fapi-pet-ct/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessment-of-myositis-related-interstitial-lung-disease-by-%e2%81%b6%e2%81%b8-ga-data-sa-fapi-pet-ct/