Session Information
Session Type: Poster Session B
Session Time: 5:00PM-6:00PM
Background/Purpose: Suboptimal medication adherence is a widespread problem in JIA. There are several unique features to medication adherence in JIA, including that the medications used are 1) primarily injectable or intravenous with varying administration frequencies, 2) difficult to safely dispose of if unused, and 3) quite expensive. Our pediatric rheumatology practice is currently developing a standardized method for assessing and addressing medication adherence barriers in patients with JIA during clinical care. Through a pilot phase of a quality improvement (QI) initiative, we aimed to test the feasibility of the Barrier Assessment Tool (BAT), created by the Pediatric Rheumatology Care and Outcomes Improvement Network (PR-COIN), within our local clinic setting and to explore perceived barriers and facilitators of adherence among a small sample of patients with JIA and their caregivers to inform future QI and qualitative work, with the long-term goal of improving adherence and minimizing the burden of treatment.
Methods: This single-center, pilot phase, QI study leveraged outpatient visit-level data from a large pediatric rheumatology clinic between May and December 2022 to assess medication adherence in JIA. One provider piloted a brief interview script to prompt discussion about medication adherence during routine clinical visits among a convenience sample of children with JIA and their caregivers. The BAT was administered to caregivers at the same visit.
We retrospectively reviewed responses to adherence interviews and the BAT and manually abstracted clinical information. We reviewed the interview text documented within the visit note to describe responses and summarize commonly reported themes.
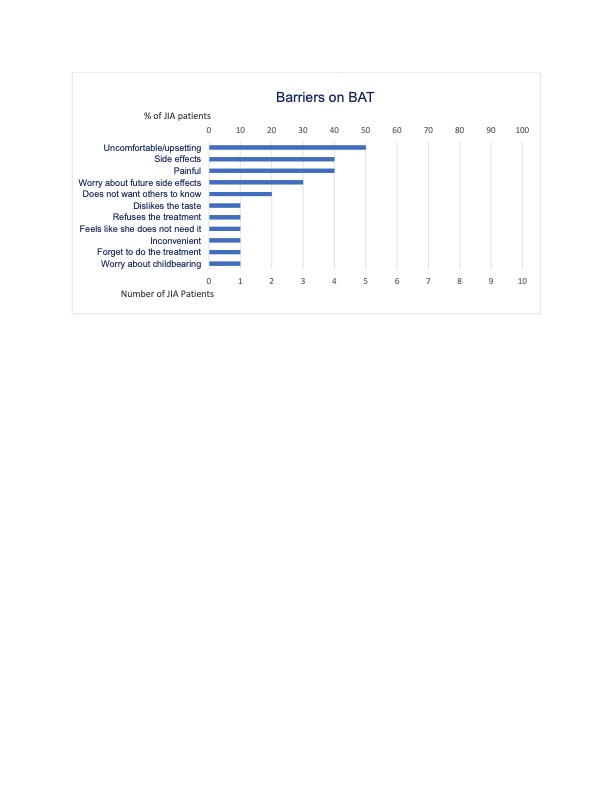
Results: A total of 10 patients were included in this study (Table 1). Patients were primarily white females with oligoarticular JIA on subcutaneous medications. Only 30% reported recent missed doses. Most patients reported at least 1 barrier (Figure 1). Patients completed the BAT with ease and expressed the importance of discussing medication adherence.
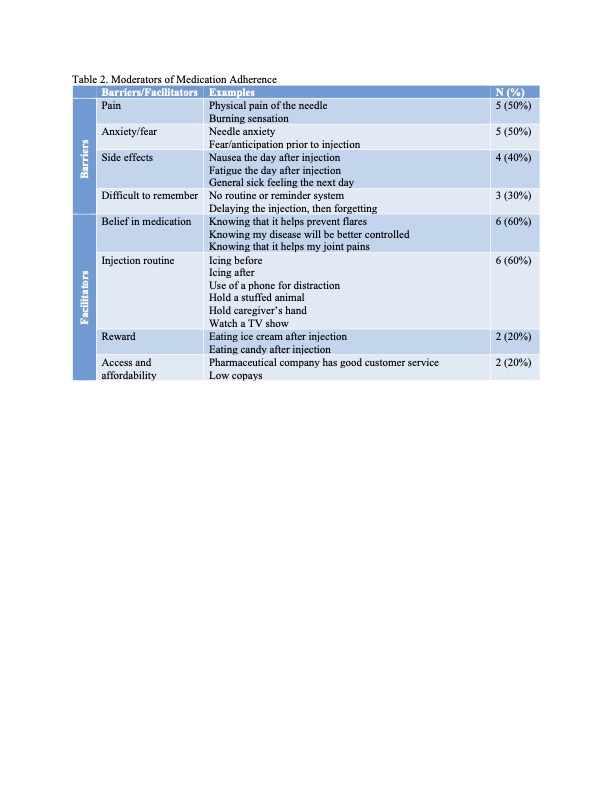
Table 2 highlights emergent themes from the interviews regarding hypothesized moderators of medication adherence. Barriers included pain and anxiety related to injections, side effects, and forgetfulness. Facilitators included belief that the medication is helpful, injection routines, food rewards, and ease of access.
Conclusion: Our findings suggest that, despite few reports of missed doses, patients with JIA experience significant challenges associated with taking their medications and are interested in discussing them. Patients reported various factors impacting adherence and only some were interested in peer support, suggesting that interventions to address adherence and treatment burden may not be a one-size-fits-all approach.
Given that self-reported adherence is known to overestimate true adherence, our findings should be validated with pharmacy fill data. Our study team aims to conduct a larger study using fill data to measure adherence combined with semi-structured interviews to further explore mechanisms influencing adherence. Future QI work will focus on assessing and addressing adherence barriers on a larger, division-wide scale.
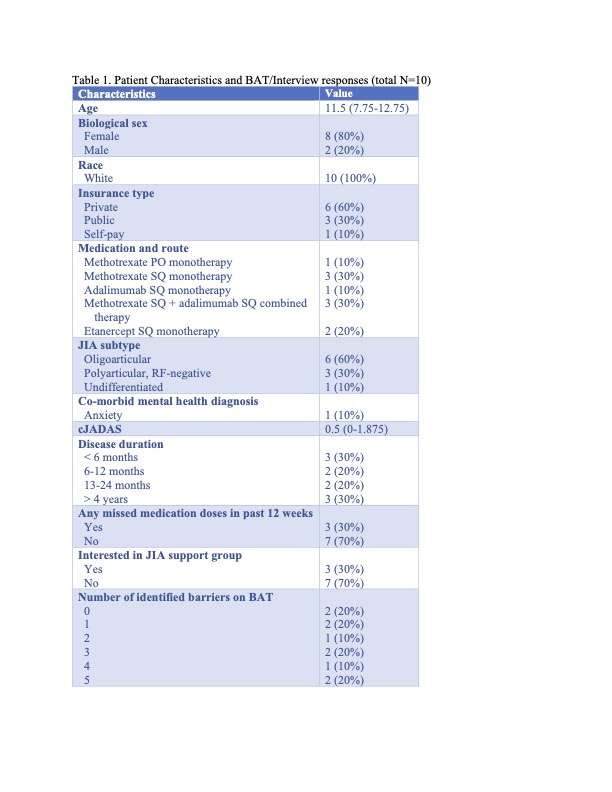 Categorical values are reported as counts and percentages. Continuous variables are reported as medians and interquartile ranges (IQR).
Categorical values are reported as counts and percentages. Continuous variables are reported as medians and interquartile ranges (IQR).
To cite this abstract in AMA style:
Abel D, Chang J, Burnham J, Kenyon C, Gmuca S. Assessing Medication Adherence in JIA: Pilot Phase Results from a Single-Center Quality Improvement Initiative [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 4). https://acrabstracts.org/abstract/assessing-medication-adherence-in-jia-pilot-phase-results-from-a-single-center-quality-improvement-initiative/. Accessed .« Back to 2023 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/assessing-medication-adherence-in-jia-pilot-phase-results-from-a-single-center-quality-improvement-initiative/