Session Information
Date: Friday, November 6, 2020
Title: Epidemiology & Public Health Poster I: COVID-19 & Rheumatic Disease
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Early in the COVID-19 pandemic, hydroxychloroquine and chloroquine were empirically promoted and used for treatment and prevention of SARS-CoV-2 infection. The repurposing of these drugs before robust efficacy data were available led to potentially harmful shortages for people with rheumatic diseases. The aims of this study were to assess (1) whether the use of antimalarials in patients with rheumatic disease was associated with a lower risk of COVID-19 infection, and (2) the prevalence and impact of drug shortages during the COVID-19 pandemic.
Methods: The COVID-19 Global Rheumatology Alliance (C19-GRA) Patient Experience Survey was distributed online through patient support organizations and on social media. Patients with rheumatic diseases (or the parents of pediatric patients) anonymously entered data including their rheumatic disease diagnosis, medications, COVID-19 status, and disease outcomes. Impact of drug shortages was evaluated for the effect on patient disease activity, mental health and physical health states by comparing mean values with two-sided independent t-tests to identify significant differences.
Results: From 9,393 respondents (mean age 46.1 (SD 12.8) years, 90.0% female), 3,872 (41.2%) were taking antimalarials (Table 1). Of these, 230 (6.2%) were unable to continue taking antimalarials because of a lack of supply at their pharmacy. 21.4% of patients in South-East Asia and 26.7% in African regions reported an inadequate supply of antimalarials in pharmacies, in contrast to 6.8% of patients in the Americas and 2.1% in European regions.
There were similar rates of COVID-19 infection among patients on antimalarials as compared to patients not on these drugs (6.7% vs. 4.7%). A total of 28 patients (10.8%) with COVID-19 who were taking antimalarials were hospitalized. Of 519 patients diagnosed with COVID-19, 68 (13.1%) indicated they were prescribed antimalarials as a treatment for their COVID-19 infection.
Patients who were unable to obtain antimalarials from their pharmacies compared to those who did not experience medication shortages experienced higher levels of rheumatic disease activity (5.1 > 4.3, t(244) = 4.44, p < 0.001) (Figure 1) and poorer mental (5.8 < 6.3, t(252) = 3.82, p < 0.001) and physical health (5.6 < 6.4, t(254) = 5.97, p < 0.001) (Figure 2).
Conclusion: Patients in African and South-East Asian regions reported greater difficulty obtaining antimalarial drugs to treat their rheumatic disease in contrast to patients in the Americas and European regions. Patients who experienced antimalarial drug shortages reported worse mental and physical health outcomes than those able to obtain their medications. Antimalarials did not protect patients with rheumatic disease from COVID-19 or from hospitalization as a result of COVID-19. The unintended harmful consequences of repurposing antimalarials, without adequate evidence for benefit, highlights the importance of maintaining scientific rigor even in the context of a pandemic. Regional disparities of access to medications should be addressed to ensure all people, particularly those living in developing countries, receive fair and equitable access to these essential medications.
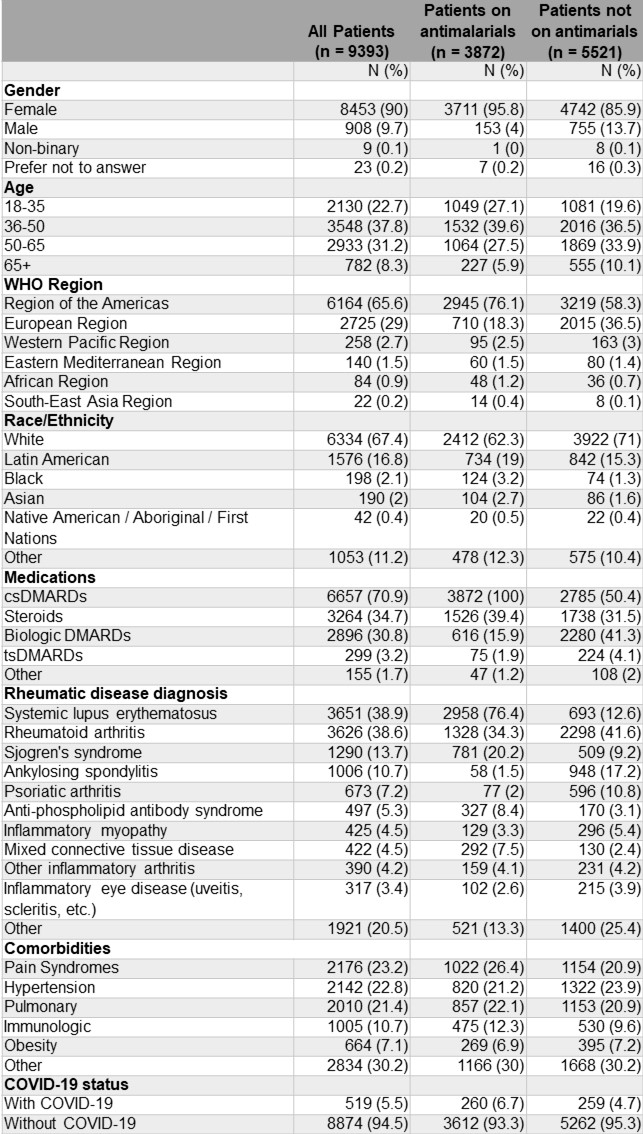 Table 1. Demographic and clinical characteristics of adults according to use of antimalarials in the C19-GRA Patient Experience Survey (n=9393). Participants may have more than one condition and take more than one type of medication. csDMARD medications included: antimalarials (hydroxychloroquine, chloroquine), azathioprine, cyclophosphamide, cyclosporine, leflunomide, methotrexate, mycophenolate mofetil/mycophenolic acid, sulfasalazine, tacrolimus. bDMARD included: abatacept, belimumab, CD-20 inhibitors, IL-1 inhibitors, IL-6 inhibitors, IL-12/IL-23 inhibitors, IL-17 inhibitors, and anti-TNF. tsDMARD included: Janus Kinase inhibitors. Other included: IVIG, apremilast, thalidomide. bDMARD, biologic DMARD; csDMARD, conventional synthetic DMARD; tsDMARD targeted synthetic DMARD, DMARD, disease-modifying antirheumatic drug.
Table 1. Demographic and clinical characteristics of adults according to use of antimalarials in the C19-GRA Patient Experience Survey (n=9393). Participants may have more than one condition and take more than one type of medication. csDMARD medications included: antimalarials (hydroxychloroquine, chloroquine), azathioprine, cyclophosphamide, cyclosporine, leflunomide, methotrexate, mycophenolate mofetil/mycophenolic acid, sulfasalazine, tacrolimus. bDMARD included: abatacept, belimumab, CD-20 inhibitors, IL-1 inhibitors, IL-6 inhibitors, IL-12/IL-23 inhibitors, IL-17 inhibitors, and anti-TNF. tsDMARD included: Janus Kinase inhibitors. Other included: IVIG, apremilast, thalidomide. bDMARD, biologic DMARD; csDMARD, conventional synthetic DMARD; tsDMARD targeted synthetic DMARD, DMARD, disease-modifying antirheumatic drug.
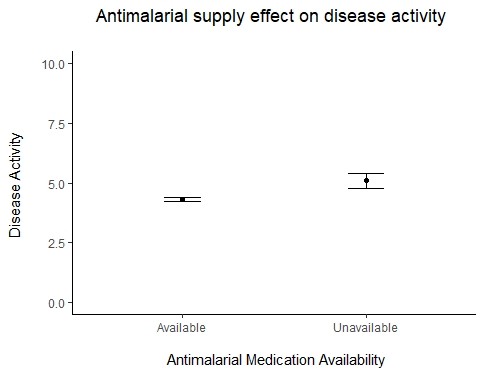 Figure 1. Antimalarial supply effect on disease activity in rheumatic disease patients.
Figure 1. Antimalarial supply effect on disease activity in rheumatic disease patients.
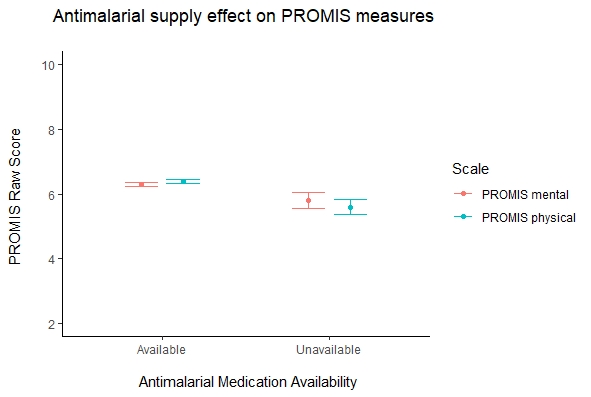 Figure 2. Antimalarial supply effect on PROMIS measures in rheumatic disease patients. Disclaimer: The views expressed here are those of the authors and participating members of the COVID-19 Global Rheumatology Alliance and do not necessarily represent the views of the American College of Rheumatology, the European League Against Rheumatism (EULAR), or any other organization.
Figure 2. Antimalarial supply effect on PROMIS measures in rheumatic disease patients. Disclaimer: The views expressed here are those of the authors and participating members of the COVID-19 Global Rheumatology Alliance and do not necessarily represent the views of the American College of Rheumatology, the European League Against Rheumatism (EULAR), or any other organization.
To cite this abstract in AMA style:
Sirotich E, Kennedy K, Surangiwala S, Semalulu T, Larche M, Liew J, Wallace Z, Robinson P, Grainger R, Sparks J, Simard J, Yazdany J, Gore-Massy M, Howard R, Levine M, Hausmann J. Antimalarial Drug Shortages During the COVID-19 Pandemic: Results from the Global Rheumatology Alliance Patient Experience Survey [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/antimalarial-drug-shortages-during-the-covid-19-pandemic-results-from-the-global-rheumatology-alliance-patient-experience-survey/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/antimalarial-drug-shortages-during-the-covid-19-pandemic-results-from-the-global-rheumatology-alliance-patient-experience-survey/
