Session Information
Date: Tuesday, November 14, 2023
Title: (2177–2194) Sjögren’s Syndrome – Basic & Clinical Science Poster II
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Extraglandular manifestations may occur in up to 40-50% of patients with SS, including inflammatory arthralgia and chronic polyarthritis (1-3). ACPA are prototypical markers of RA, but have also been described in 4-10% of patients with SS (3,4). Although ACPA have been associated with articular and lung involvement, their clinical relevance remains to be fully clarified (3,4).
Objective: To evaluate the prevalence of ACPA in SS patients and assess their associations with clinical, laboratorial and radiographic features.
Methods: We screened patients from the Observational Lisbon Sjögren’s Syndrome Prospective (OLISSIPO) and University of Pisa (UNIPI) cohorts, included until May 2023, for the presence of ACPA. For each ACPA-positive, two ACPA-negative patients matched for age and sex were selected. We collected demographic, clinical and laboratorial variables. t-student or Mann-Whitney tests were used, as appropriate, for continuous variables and chi-square or Fisher tests for categorical variables.
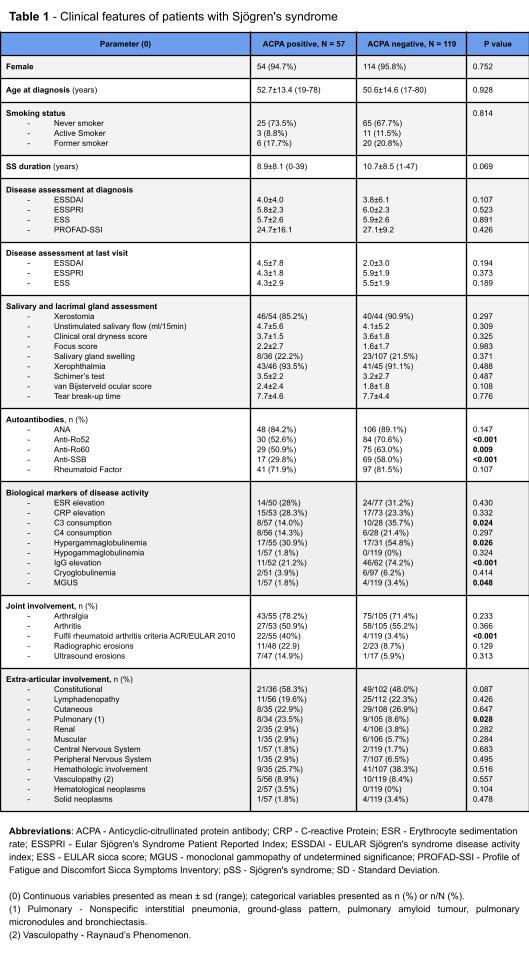
Results: From 438 patients tested for ACPA, 176 patients (95.5% women) were selected (Table 1), 57 of them ACPA-positive (94.7% female), corresponding to a prevalence of 13.0%. Age at pSS diagnosis and disease duration were similar between groups. There were no differences in systemic disease activity, as assessed by the EULAR Sjögren’s Syndrome disease activity index (ESSDAI), nor in dryness, pain and fatigue assessed by the EULAR Patient Reported Index (ESSPRI), EULAR sicca score (ESS), and Profile of Fatigue and Discomfort Sicca Symptoms Inventory (PROFAD-SSI). Similarly, xerostomia, xerophthalmia, salivary gland swelling and objective measures of reduced glandular function or inflammation (focus score) were also comparable between ACPA-positive and ACPA-negative patients. However, ACPA-positivity was associated with a significantly lower frequency of anti-Ro52 (52.6% vs 70.6%, p< 0.001), anti-Ro60 (50.9% vs 63.0%, p=0.009), and anti-SSB (29.8% vs 58.0%, p< 0.001) antibodies, but no differences in RF positivity (71.9% vs 81.5%, p=0.107). In accordance, markers of B cell hyperactivation such as C3 consumption (14.0% vs 35.7%, p=0.024), hypergammaglobulinemia (30.9% vs 54.8%, p=0.026) and IgG elevation (21.2% vs 74.2%, p< 0.001) were also less commonly associated with the presence of ACPA. Of note, articular involvement or erosive disease was not more frequent in ACPA-positive patients. Nonetheless, a higher number of ACPA-positive patients met the ACR/EULAR 2010 classification criteria for RA (40.0% vs 3.4%, p< 0.001). Finally, lung disease was significantly more common in the ACPA-positive group (23.5% vs 8.6%, p=0.028), whereas involvement of additional organs was not.
Conclusion: In patients with SS, ACPA is not associated with joint involvement, but, surprisingly, seems to define a subgroup of patients with lower markers of B cell activation (such as circulating anti-SSA/B, complement consumption and raised immunoglobulins). Furthermore, ACPA was associated with lung involvement, despite a similar distribution of known markers of pulmonary disease in SS such as RF. These findings deserve further confirmation in larger cohorts.
To cite this abstract in AMA style:
Silva A, Costa F, Silva M, Fulvio G, Bandeira M, Silvério-António M, Khmelinskii N, Baldini C, Romão V. Anti-citrullinated Protein Antibodies in Sjögren’s Syndrome Define a Subset of Patients with Lower B Cell Activation Markers and Higher Risk of Lung Involvement [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/anti-citrullinated-protein-antibodies-in-sjogrens-syndrome-define-a-subset-of-patients-with-lower-b-cell-activation-markers-and-higher-risk-of-lung-involvement/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/anti-citrullinated-protein-antibodies-in-sjogrens-syndrome-define-a-subset-of-patients-with-lower-b-cell-activation-markers-and-higher-risk-of-lung-involvement/