Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Distinguishing patients with axial spondyloarthritis (axSpA) from patients with other causes of chronic back pain remains a challenge. The lack of reliable biomarkers contributes to the diagnostic delay in axSpA. Recently, macrophage migration inhibitory factor (MIF) has been proposed as a candidate diagnostic and prognostic biomarker. MIF is a proinflammatory cytokine that was shown to be upregulated in several autoimmune/inflammatory diseases including axSpA. The putative role of CD8+ T cells in axSpA pathogenesis suggests further that serum markers of cytotoxicity might have value as biomarkers in axSpA. The goal of this study was to compare serum levels of MIF and other candidate serum proteins in patients with axSpA and controls.
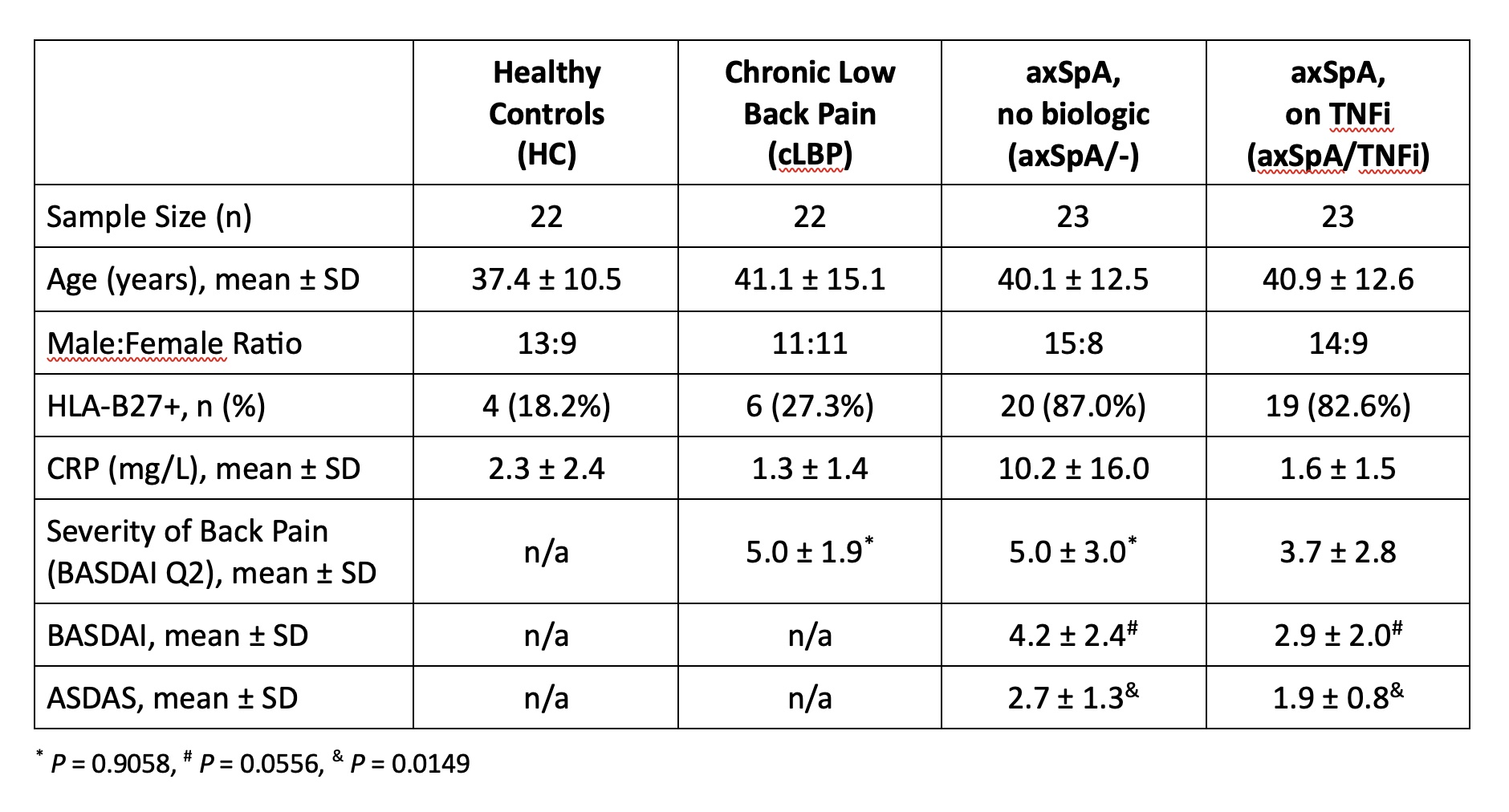
Methods: Study subjects were recruited from our hospital’s Orthopedic and Arthritis Center. Four cohorts were compared: healthy controls (HC), patients with chronic low back pain without axSpA (cLBP), axSpA patients not on a biologic (axSpA/-), and axSpA patients treated with a TNF inhibitor (axSpA/TNFi). Study subjects were matched for age, sex and genotyped for HLA-B27 (Table 1). All axSpA patients fulfilled modified New York criteria for ankylosing spondylitis or the 2009 ASAS criteria for axSpA. Serum was evaluated using the LEGENDplex Human CD8/NK panel (BioLegend) for thirteen markers including IL-17A, IL-6, TNF, granzyme B, and perforin. CRP and MIF were evaluated by DuoSet ELISA (R&D Systems).
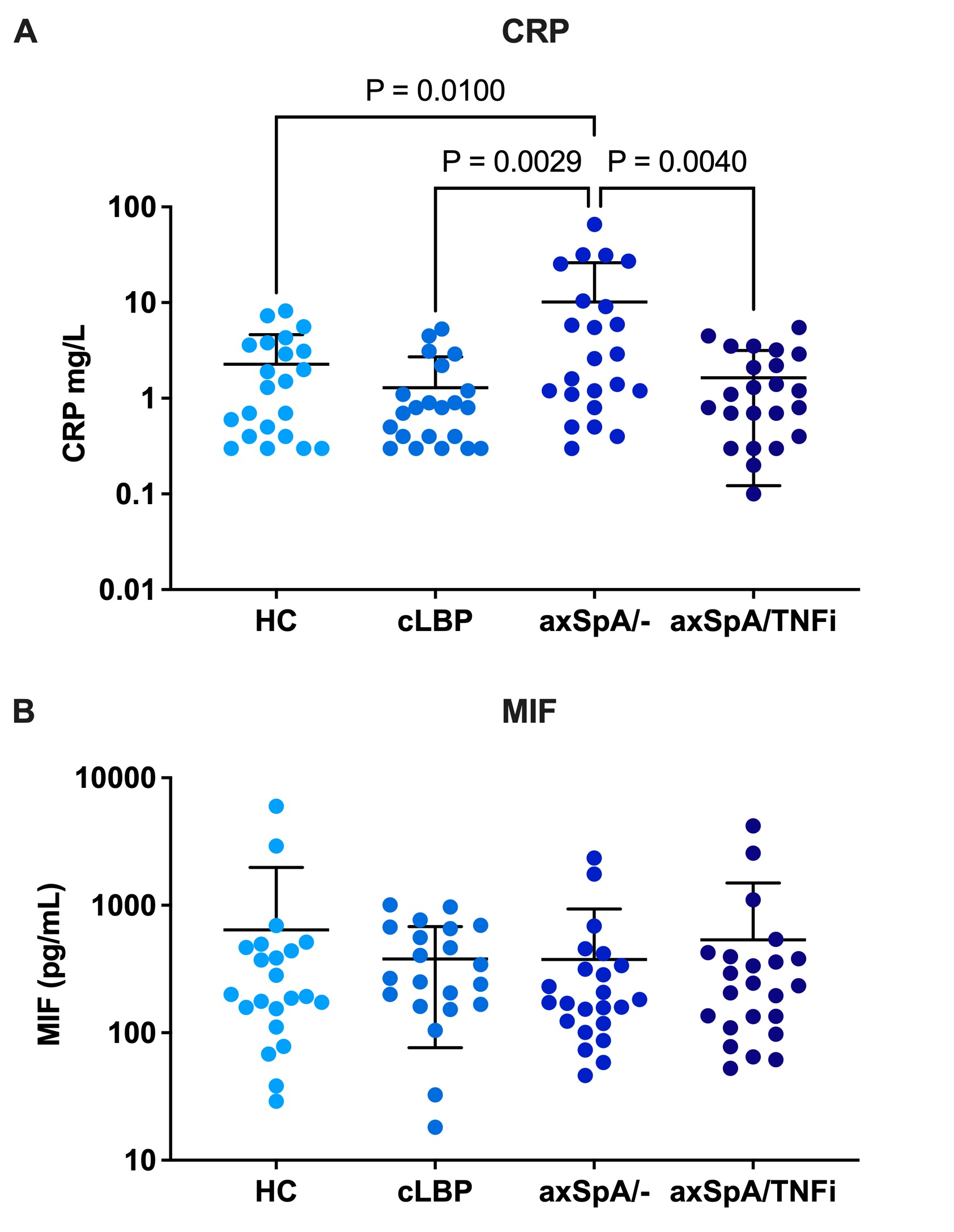
Results: The severity of back pain in the cLBP controls and axSpA/- patients was comparable (BASDAI Q2 mean 5.0±1.9 vs. 5.0± 3.0). axSpA/- patients had higher back pain, BASDAI and ASDAS scores than axSpA/TNFi patients consistent with higher disease activity in the biologic naïve group. Serum CRP values were significantly higher in axSpA/- patients compared with HC, cLBP controls, and axSpA/TNFi patients (P= 0.01, P=0.0029, P=0.004 respectively) (Figure 1A). Serum MIF levels were not statistically different across all four groups (P= 0.8069) (Figure 1B). Additionally, there were no statistically significant differences between the groups for any of the markers included in the LEGENDplex Human CD8/NK panel.
Conclusion: The strength of this study is the comparison of well-characterized groups of axSpA patients with/without biologic therapy and controls including patients with cLBP. In contrast to a previous study, we did not find differences in serum MIF levels between axSpA patients and controls. Of the evaluated serum biomarkers, only CRP values correlated with active axSpA. Our results underscore the need for more research into diagnostic biomarkers in axSpA.
To cite this abstract in AMA style:
Bauchiero C, Sinnappan S, Ermann J. Analysis of Soluble Biomarkers in Axial Spondyloarthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/analysis-of-soluble-biomarkers-in-axial-spondyloarthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/analysis-of-soluble-biomarkers-in-axial-spondyloarthritis/