Session Information
Date: Tuesday, November 9, 2021
Title: Osteoarthritis & Joint Biology – Basic Science Poster (1468–1479)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Osteoarthritis (OA) is one of the leading causes of musculoskeletal pain and disability. Worldwide, an estimated 303 million patients have clinically diagnosed OA, with over 14 million new cases emerging annually. Yet, management of OA pain remains poor, and often relies on analgesics with limited efficacy. To identify new analgesic targets, we use molecular biology techniques to study the mechanisms underlying OA pain in mouse models. Single cell RNA-sequencing of mouse dorsal root ganglia (DRG) identified novel molecular targets, including several G-protein coupled receptor (GPCR) genes with specific expression patterns in populations of neuronal and non-neuronal DRG cells. In particular, we found that the rhodopsin class GPCR, Gpr34, is specifically expressed by macrophages in the DRG. Given that neuroimmune interactions have been implicated in the development of persistent pain, targets on immune cells infiltrating the DRG may be therapeutically useful. The objective of this study was to investigate expression of Gpr34 by DRG macrophages in experimental OA, induced by partial meniscectomy (PMX). We have previously shown that both male and female mice develop joint damage and signs of pain behavior in this model by 12 weeks post surgery.
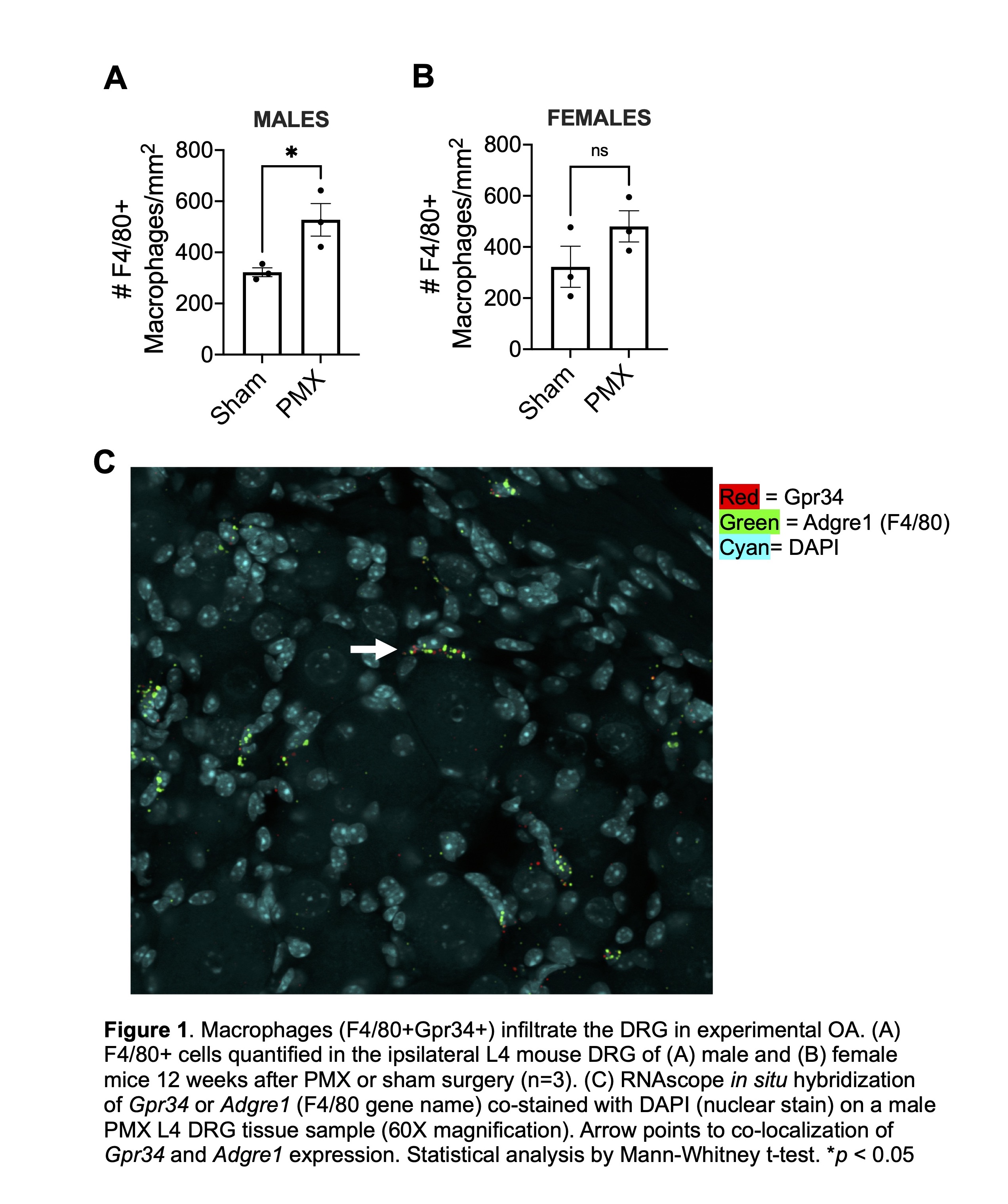
Methods: We performed PMX or sham surgery on 10-week-old male and female mice (n=3 per group). Ipsilateral L4 DRG were collected 12 weeks following surgery, fixed in 4% paraformaldehyde, transferred to 30% sucrose solution for cryoprotection and cryo-sectioned onto slides. RNAscope analysis was completed with probes for mouse Gpr34 and Adgre1 per ACD Bio-Techne Multiplex Fluorescent v2 Assay standard protocol. We evaluated macrophage presence in the DRG via immunofluorescence staining for F4/80, a pan-macrophage marker, as previously described. Final images were processed using a Fluoview FV10i confocal microscope, and quantification was completed via ImageJ software. Briefly, in ImageJ, regions of interest (ROIs) were automatically generated from a set intensity threshold and cell size parameters, and the number of positive cells in 30X image area were counted by a blinded observer.
Results: By immunofluorescence, we observed an increase in the total number of F4/80+ cells in DRGs 12 weeks post PMX compared to sham controls, in both male and female mice (n=3) (Figs. 1A+B). In addition, RNAscope in situ hybridization revealed overlap with Gpr34 and Adgre1 (gene name for F4/80) 12 weeks post PMX in male mice, supporting the single cell RNA sequencing data that these cells are indeed macrophages (n=3) (Fig. 1C).
Conclusion: This study suggests that macrophages are increased in the DRGs of both male and female mice after PMX, during a time when the mice have developed both joint damage and persistent pain behaviors. Our lab has previously shown that macrophages numbers also increase in DRGs following destabilization of the medial meniscus (DMM), suggesting that these cells may contribute to the development of persistent pain. Moreover, a novel druggable receptor, Gpr34 is expressed by these macrophages. Future work will examine the contribution of macrophages and Gpr34 to OA pain, and GPR34 and macrophage gene expression in human DRGs.
To cite this abstract in AMA style:
Thomas D, Geraghty T, Obeidat A, Li J, Miller R, Malfait A. An Orphan Receptor and Osteoarthritis: Mouse Gpr34 Expression in Dorsal Root Ganglia After Partial Meniscectomy [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/an-orphan-receptor-and-osteoarthritis-mouse-gpr34-expression-in-dorsal-root-ganglia-after-partial-meniscectomy/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/an-orphan-receptor-and-osteoarthritis-mouse-gpr34-expression-in-dorsal-root-ganglia-after-partial-meniscectomy/