Session Information
Date: Sunday, November 8, 2020
Title: RA – Treatments Poster III: PROs, Biomarkers, Systemic Inflammation & Radiographs
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Methotrexate (MTX) can result in an increase in mean corpuscular volume (MCV) of red blood cells. The range of MCV change varies between patients on treatment with MTX. We investigated MCV as a biomarker of response to MTX treatment in early rheumatoid arthritis (RA) patients.
Methods: This is a real-world data of biologic-naïve RA patients (as per 2010 ACR/EULAR classification) in a teaching hospital who were started on oral MTX. Results were validated by using a second cohort from a different hospital. Treatment response to oral MTX was defined as at least moderate to good EULAR response and no initiation of additional conventional, synthetic or biologic disease-modifying agents within 6 months. After initiation of MTX, MCV changes were analysed (stratified by responder and non-responder groups), by using linear mixed model analysis. Receiver operating characteristic (ROC) curve analysis (area under the curve [AUC]) was employed to identify the optimal cut-point of MCV change. Kaplan-Meier analysis captured the cumulative adverse drug reactions (ADRs) over 6 months due to MTX. Multivariate logistic regression was applied to identify predictors of treatment response within patient demographics, baseline characteristics, antibody status and concomitant treatment. Latent classes were then identified based on MCV changes.
Results: Although the MCV increased from baseline to 3 months and beyond in both responders and non-responders, MTX-responder showed a significantly greater increase of MCV from 3 months post-MTX compared to non-responders (p< 0.001, Fig. 1A). This finding was validated in a second cohort (Fig. 1B). An increase in MCV of 4 femtolitres (fL) at 3 months was associated with better treatment response using a ROC analysis with an AUC of 0.76 (95% CI 0.73- 0.80). AUC was 0.75 (95% CI 0.69- 0.83) for increase in MCV by 4.2 fL in the second cohort. However, a raised MCV ≥ 4 fL was not associated with increased ADRs (Fig. 1C). Hydroxychloroquine (HCQ) therapy potentiated the MCV changes when combined with MTX, in both treatment responder and non-responder group (Fig. 1D). There was no change in MCV when HCQ was given without methotrexate suggesting a synergistic effect with MTX (data not shown).
Multivariate logistic regression revealed an increment of MCV by ≥ 4 fL [Odds Ratio (OR), 3.97 with 95% CI of 2.92-5.42; p=0.001] and concomitant use of HCQ (OR 2.17 with 95% CI 1.61-2.93; p=0.003) predicted clinical response (Fig. 2).
Two latent classes were identified based on MCV changes (Fig. 3): a greater increase in MCV group (class 1) which was associated with more treatment responders [Log (OR) 5.93, SE 0.85, p=0.001)] compared to the other class (class 2) with minimal increase in MCV. Higher MCV over time in class 1 was associated with lower C-reactive protein (CRP) [Log (OR) 0.97, SE 0.24, p=0.001] and concomitant use of HCQ [Log (OR) 0.28, SE 0.7, p=0.000)]. This effect was in the opposite direction for class 2 [Log (OR) for CRP was 0.33 with SE 0.04, p=0.000 and for HCQ -0.38 with SE 0.07, p=0.000].
Conclusion: Our data suggests that an increase of MCV increase of 4 fL from 3 months predicts response to MTX therapy in biologic-naïve RA patients and that concomitant HCQ potentiates the increase in MCV and the therapeutic effect of MTX.
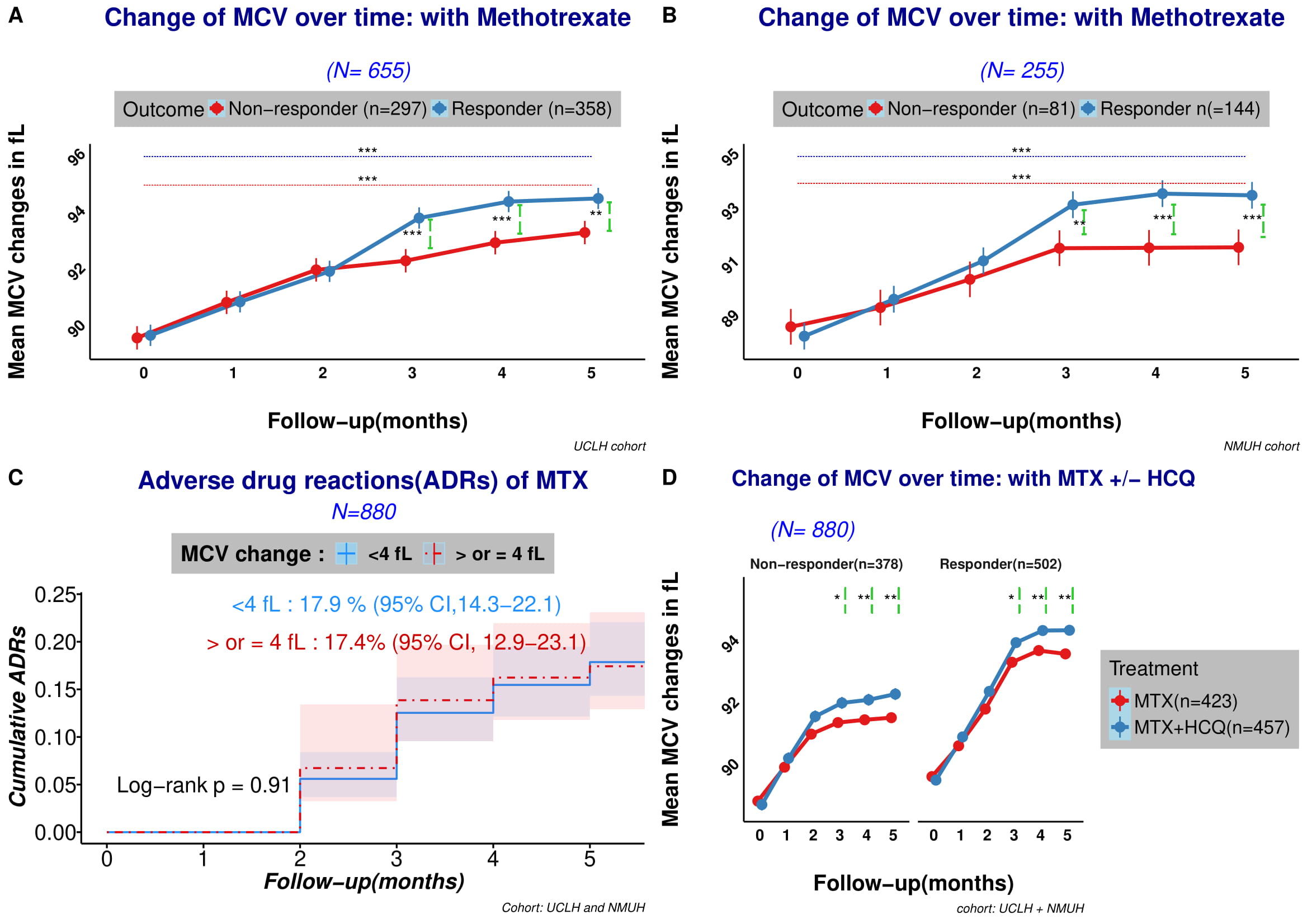 Figure 1 (A-D): A. MCV (mean corpuscular volume) change after initiation of methotrexate (MTX). Overall, MCV increased significantly from baseline for both MTX responder and non-responder groups. A significantly higher increase in MCV is demonstrated in the responder group from month 3 and onwards. B. The changes in MCV seen in 1A were confirmed in a second cohort of patients. C. Kaplan-Meier survival curves of adverse drug reactions (ADRs) event in the first 6 months after initiation of methotrexate (MTX) stratified by mean corpuscular volume (MCV) change (either < 4 or ≥ 4 fL) from baseline did not reveal any difference. Overall adverse effect was noted for 17.4% (95% CI 12.9-23.1) of patient who had an MCV increment of ≥ 4 fL, compared to 17.9% who had an MCV increase of < 4 (95% CI 14.3-22.1). Both cohorts have been combined for ADRs. ADRs include: MCV change < 4: 20 deranged liver function (10 resulting in drug discontinuation), 2 suspected pneumonitis, 2 infections, 1 pancytopenia, 29 gastrointestinal (GI) side effects, 3 other causes. MCV ≥ 4: 31 deranged liver function (13 resulting in drug discontinuation), 33 GI side effects, 2 thrombocytopenia, 1 hypersensitivity reaction, 13 other causes. D. adding hydroxychloroquine (HCQ) to MTX potentiated the MCV changes. Each error bar (lime green) represents the p value between MTX monotherapy patient and MTX+HCQ group each time point.
Figure 1 (A-D): A. MCV (mean corpuscular volume) change after initiation of methotrexate (MTX). Overall, MCV increased significantly from baseline for both MTX responder and non-responder groups. A significantly higher increase in MCV is demonstrated in the responder group from month 3 and onwards. B. The changes in MCV seen in 1A were confirmed in a second cohort of patients. C. Kaplan-Meier survival curves of adverse drug reactions (ADRs) event in the first 6 months after initiation of methotrexate (MTX) stratified by mean corpuscular volume (MCV) change (either < 4 or ≥ 4 fL) from baseline did not reveal any difference. Overall adverse effect was noted for 17.4% (95% CI 12.9-23.1) of patient who had an MCV increment of ≥ 4 fL, compared to 17.9% who had an MCV increase of < 4 (95% CI 14.3-22.1). Both cohorts have been combined for ADRs. ADRs include: MCV change < 4: 20 deranged liver function (10 resulting in drug discontinuation), 2 suspected pneumonitis, 2 infections, 1 pancytopenia, 29 gastrointestinal (GI) side effects, 3 other causes. MCV ≥ 4: 31 deranged liver function (13 resulting in drug discontinuation), 33 GI side effects, 2 thrombocytopenia, 1 hypersensitivity reaction, 13 other causes. D. adding hydroxychloroquine (HCQ) to MTX potentiated the MCV changes. Each error bar (lime green) represents the p value between MTX monotherapy patient and MTX+HCQ group each time point.
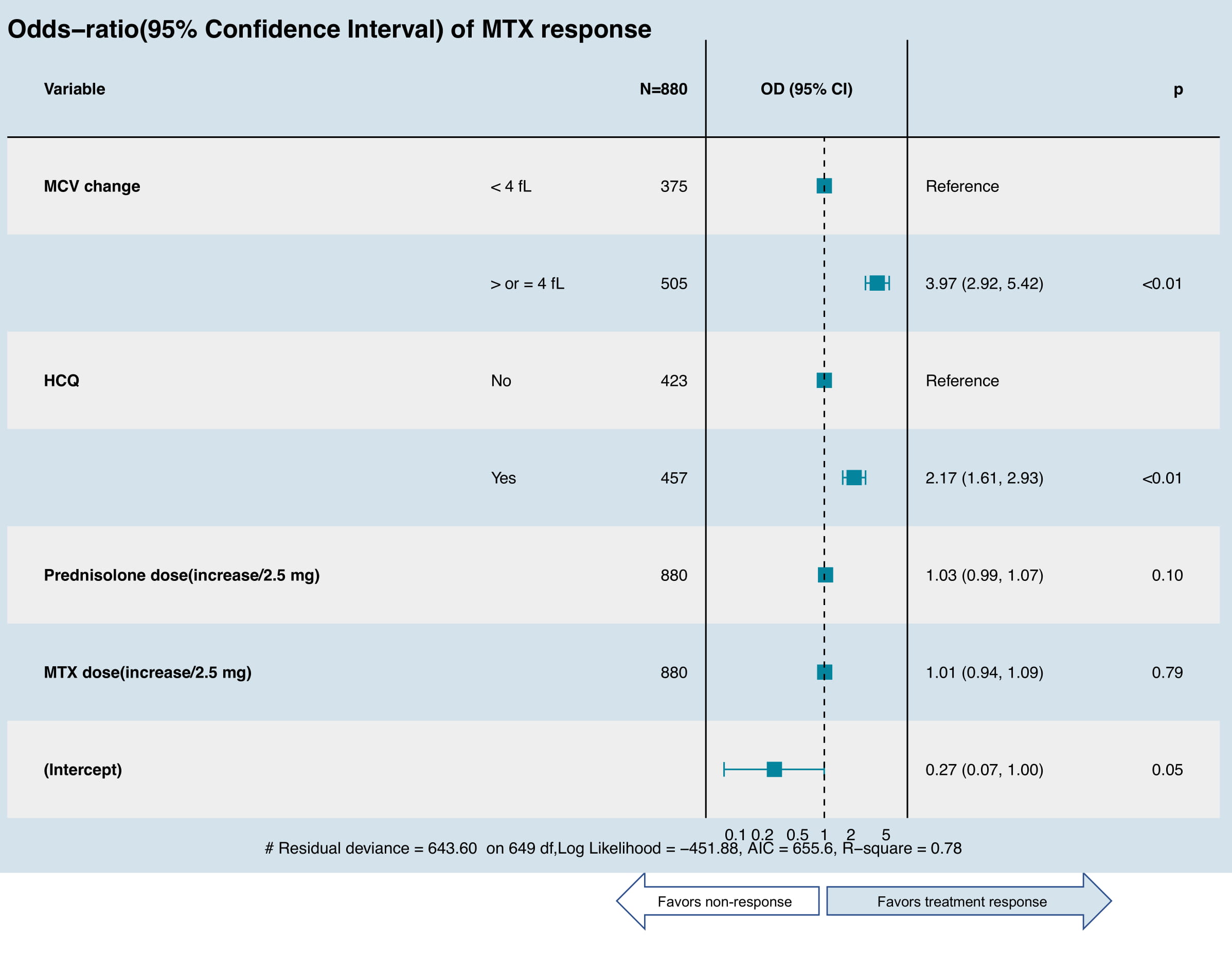 Figure 2: Random- and fixed-effects multivariate logistic regression model of predictors of treatment response of methotrexate (MTX). The final model was selected by stepwise regression using Akaike information criterion (AIC), which included: – Change of MCV (used as categorial variable), disease duration (not shown, not significant in the final model) – Adjusted by concomitant prednisolone (if applicable), MTX dose, and concomitant use of disease-modifying antirheumatic drugs (DMARDs) which includes hydroxychloroquine (HCQ), sulfasalazine (SSZ) or leflunomide (LEF).
Figure 2: Random- and fixed-effects multivariate logistic regression model of predictors of treatment response of methotrexate (MTX). The final model was selected by stepwise regression using Akaike information criterion (AIC), which included: – Change of MCV (used as categorial variable), disease duration (not shown, not significant in the final model) – Adjusted by concomitant prednisolone (if applicable), MTX dose, and concomitant use of disease-modifying antirheumatic drugs (DMARDs) which includes hydroxychloroquine (HCQ), sulfasalazine (SSZ) or leflunomide (LEF).
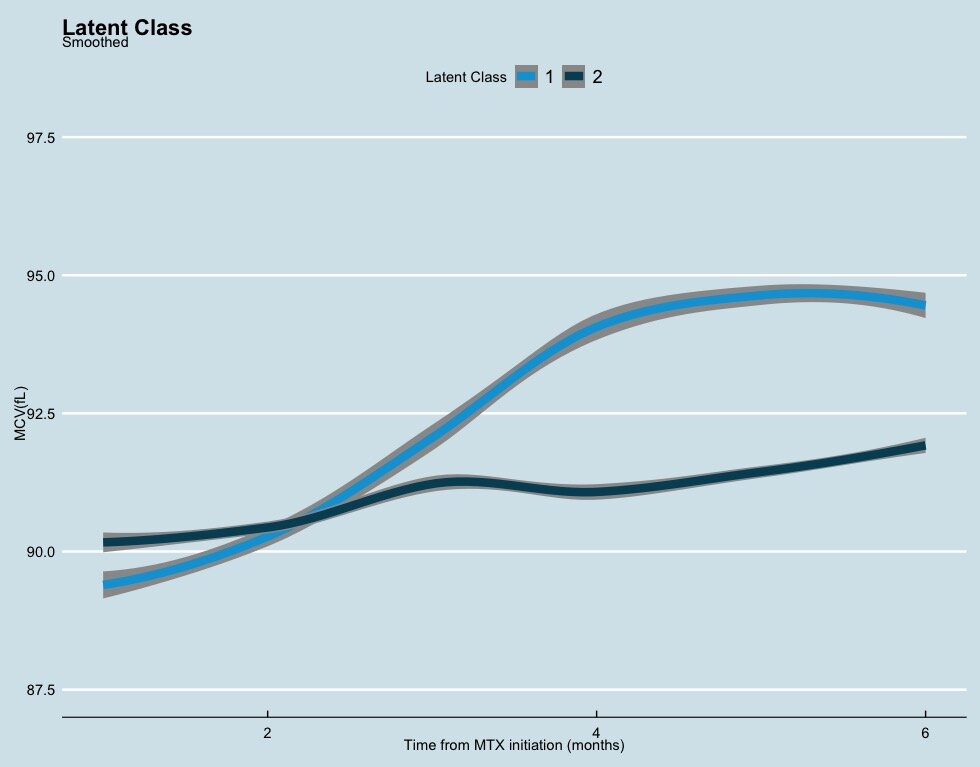 Figure 3: Mean profile change of mean corpuscular volume (MCV) over 6 months from initiation of methotrexate (MTX) after combining both cohorts and stratified by predicted class membership. Class 1: greater increase in MCV (light blue): N=496, 56.64% Class 2: minimal increase in MCV (dark blue): N=384, 43.63%. (entropy – 0.9863)
Figure 3: Mean profile change of mean corpuscular volume (MCV) over 6 months from initiation of methotrexate (MTX) after combining both cohorts and stratified by predicted class membership. Class 1: greater increase in MCV (light blue): N=496, 56.64% Class 2: minimal increase in MCV (dark blue): N=384, 43.63%. (entropy – 0.9863)
To cite this abstract in AMA style:
Shipa M, Yeoh S, Mukerjee D, Ehrenstein M. An Increase in Red Cell Mean Corpuscular Volume by Methotrexate Is Potentiated by Hydroxychloroquine and Predicts Clinical Response in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/an-increase-in-red-cell-mean-corpuscular-volume-by-methotrexate-is-potentiated-by-hydroxychloroquine-and-predicts-clinical-response-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/an-increase-in-red-cell-mean-corpuscular-volume-by-methotrexate-is-potentiated-by-hydroxychloroquine-and-predicts-clinical-response-in-rheumatoid-arthritis/
