Session Information
Date: Monday, November 9, 2020
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster III: Bench to Bedside
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Current technologies do not support long-term cell viability, differentiation and maintenance of podocytes. We developed a biophysical approach, termed macromolecular crowding (MMC), to create extracellular matrix (ECM)-rich tissue equivalents and decellularization. This approach generates decellularized grafts that scaffold podocytes to grow in an environment similar to native conditions. To show a potential application of this newly designed culture system we studied complement (C) activation in podocytes exposed to IgG from individuals with lupus nephritis (LN) and hypoxia.
Methods: Human skin fibroblasts were cultured under MMC and then decellularized. Human immortalized podocytes were cultured on the decellularized matrix (DCM) at 33°C for 7 days and at 37°C for 14 days (https://doi.org/10.1002/adfm.201908752). ECM deposition in the DCM-coated dishes was analyzed by SDS-PAGE, immunofluorescence (IF) and atomic force microscopy and expression of podocyte markers by western blotting (WB) and IF. Podocytes were then exposed to IgG from patients with LN or Dimethyloxalylglycine (DMOG) and C activation was studied (WB and IF).
Results: We found that DCM-coated dishes contained all major ECM molecules (laminin, fibronectin, collagen I and IV) and podocytes survived and differentiated on DCM-coated plates significantly better than on non-coated plates, as shown by the development of interdigitating foot process and increased expression of nephrin and synaptopodin (fig. 1). Podocytes exposed to LN IgG (fig. 2) or DMOG (fig. 3) displayed increased levels of complement molecules (C3, C4, C5, C5b9) and C3 activation products.
Conclusion: Engineering in vitro microenvironment with decellularized matrix enhances podocyte viability, native physiology and morphology. This novel system enabled us to demonstrate increased complement factor production by podocytes exposed to LN IgG or hypoxia and intracellular complement activation.
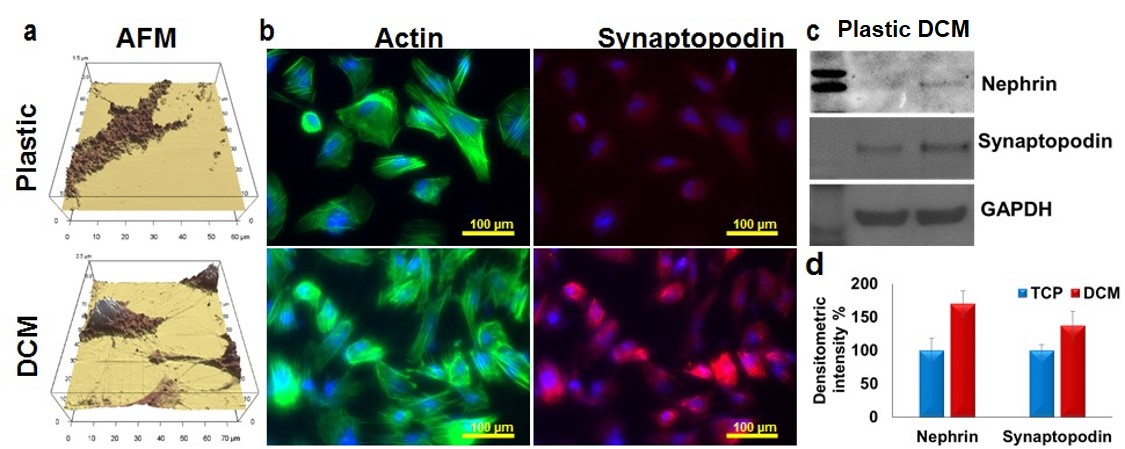 (a) Atomic Force Microscopy (AFM) shows better podocyte morphology with interdigitating foot processes on DCM-coated dishes. (b) Immunofluorescence shows enhanced expression and co-localization of actin and synaptopodin on DCM-coated dishes. Western blotting (c) (densitometric readings d,) confirmed higher expression of nephrin and synaptopodin on decellularized ECM rich substrate.
(a) Atomic Force Microscopy (AFM) shows better podocyte morphology with interdigitating foot processes on DCM-coated dishes. (b) Immunofluorescence shows enhanced expression and co-localization of actin and synaptopodin on DCM-coated dishes. Western blotting (c) (densitometric readings d,) confirmed higher expression of nephrin and synaptopodin on decellularized ECM rich substrate.
 Lupus IgG enhances production of activated C3 molecules. Lupus IgG (L IgG), Normal IgG (N IgG).
Lupus IgG enhances production of activated C3 molecules. Lupus IgG (L IgG), Normal IgG (N IgG).
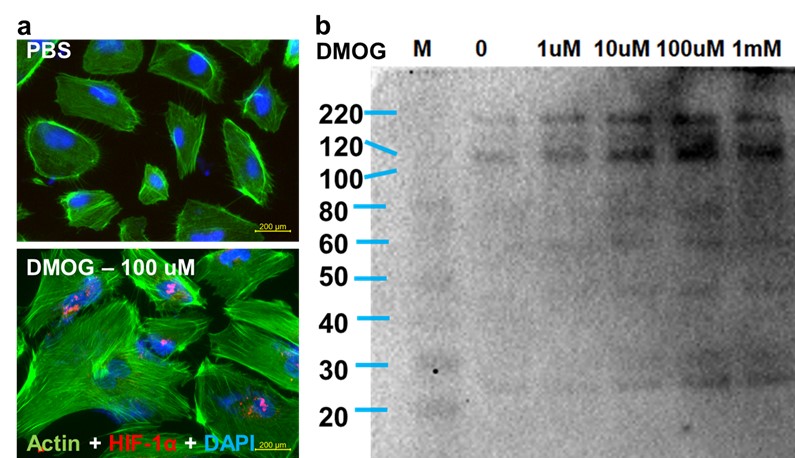 (b) DMOG enhances production of activated C3 molecules.Exposure of podocytes to DMOG (a) upregulates expression of HIF-1α and (b) enhances production of C3 split products. Western blotting analysis of activated C3 using a pan-C3 antibody that detects activated C3 split molecules.
(b) DMOG enhances production of activated C3 molecules.Exposure of podocytes to DMOG (a) upregulates expression of HIF-1α and (b) enhances production of C3 split products. Western blotting analysis of activated C3 using a pan-C3 antibody that detects activated C3 split molecules.
To cite this abstract in AMA style:
Satyam A, Tsokos M, Tsokos G. An Engineered Extracellular Matrix‐rich Decellularized Substrate Based Podocytes Culture System to Study Intracellular Complement Production and Activation [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/an-engineered-extracellular-matrix%e2%80%90rich-decellularized-substrate-based-podocytes-culture-system-to-study-intracellular-complement-production-and-activation/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/an-engineered-extracellular-matrix%e2%80%90rich-decellularized-substrate-based-podocytes-culture-system-to-study-intracellular-complement-production-and-activation/
