Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Cutaneous lupus erythematosus (CLE) can present in association with or without concomitant SLE, and with skin manifestations varying by subtype – acute CLE, subacute CLE (SCLE), and chronic CLE, of which the most common type is discoid (DLE). There is evidence of heterogeneity of response to therapy between subtypes, and surveys have shown that providers have preference for prescribing different medications based on subtype. Additionally, studies have shown that black patients are disproportionately affected by lupus, have a higher risk of damage, and have a higher incidence of CLE (specifically DLE) than white patients. However, there is a lack of evidence-based studies comparing medication response by subtype or race.
Methods: In this retrospective study of our longitudinal prospective database, we examined response rates to methotrexate (MTX), mycophenolate mofetil (MMF), and azathioprine (AZA), the most commonly used immunosuppressive medications in our cohort, by subtype and by race. Response or non-response was determined by 50% improvement or lack thereof in skin activity measured by the Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) and/or chart abstraction, and analyzed using Fisher’s exact test.1 Discontinuations due to side effects were analyzed separately.
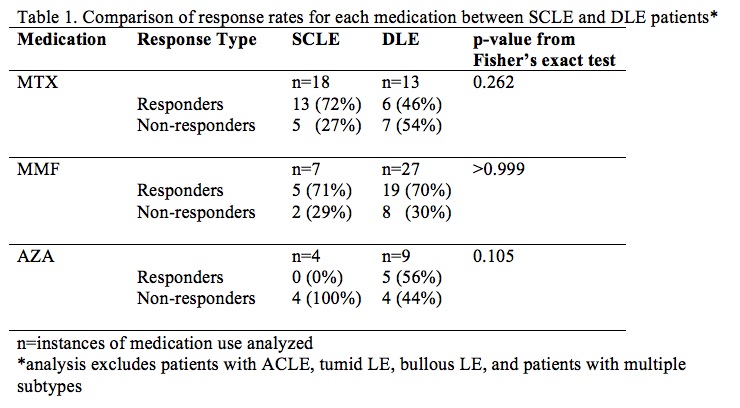
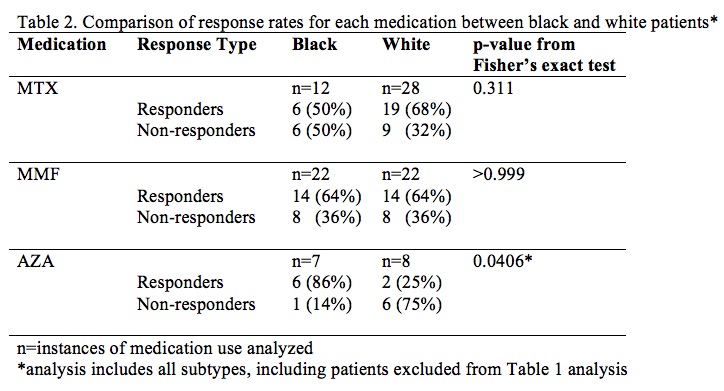
Results: Of the 191 patients with SCLE and DLE identified with sufficient longitudinal follow-up, 73 took at least one of these medications, including 34 with concomitant SLE by ACR 1997 criteria; the other subtypes were not powered for analysis and were excluded. Mean response rate was 52.6% (95% CI 30.3-74.9%). No statistically significant difference was found in rate of response to each medication when comparing SCLE vs DLE (p > 0.05). Results suggest that MMF may be more effective in DLE than MTX, but our sample was not powered to show this difference (p=0.175). Additionally, response rates were analyzed between black CLE patients and white CLE patients taking these medications. Of the 97 black CLE patients identified, 38 took at least one of these medications, including 19 with concomitant SLE by ACR 1997 criteria; of the 159 white, 53 did, including 21 with concomitant SLE by ACR 1997 criteria. Mean response rate was 59.3 (95% CI 43-75.6%). No statistically significant difference was found in rate of response to MTX or MMF in black vs white CLE patients (p > 0.05). Rate of response to AZA was higher in black patients than white patients (p = 0.0406).
Conclusion: These findings suggest that MTX and MMF appear equally efficacious between SCLE and DLE patients and between black and white patients, and AZA may work better in black patients than in white CLE patients. Further studies are needed to assess whether MMF may work better than MTX in DLE. Clinicians may then use other factors to guide medication choice, such as side effect profile and comorbid conditions.
1. Chakka S, Krain RL, Ahmed S, et al. Evaluating change in disease activity needed to reflect meaningful improvement in quality of life for clinical trials in cutaneous lupus erythematosus. J Am Acad Dermatol. 2021 Jun;84(6):1562-1567.
To cite this abstract in AMA style:
Keyes E, Jobanputra A, Grinnell M, Feng R, Vazquez T, Diaz D, Werth V. An Analysis of Medication Responsiveness Based on Subtype and Race Within a Cohort of Cutaneous Lupus Erythematosus Patients [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/an-analysis-of-medication-responsiveness-based-on-subtype-and-race-within-a-cohort-of-cutaneous-lupus-erythematosus-patients/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/an-analysis-of-medication-responsiveness-based-on-subtype-and-race-within-a-cohort-of-cutaneous-lupus-erythematosus-patients/