Session Information
Date: Sunday, November 10, 2019
Title: SLE – Animal Models Poster
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: CD6 is a co-stimulatory receptor on T cells, that binds to activated leukocyte cell adhesion molecule (ALCAM), a ligand expressed on antigen presentation cells and epithelial and endothelial tissues. The CD6/ALCAM pathway plays an integral role in modulating T cell activation and trafficking, making this an integral pathway in immuno-inflammation. Increased levels of CD6 are associated with pathogenic T cell responses, suggesting that the CD6/ALCAM pathway is a contributor to disease pathogenesis. T cells are a key cellular mediator in both systemic lupus erythematosus (SLE) and lupus nephritis (LN), as they amplify the autoimmune response and inflammation and contribute to end organ damage. Thus, we designed an experiment to target them via CD6 modulation.
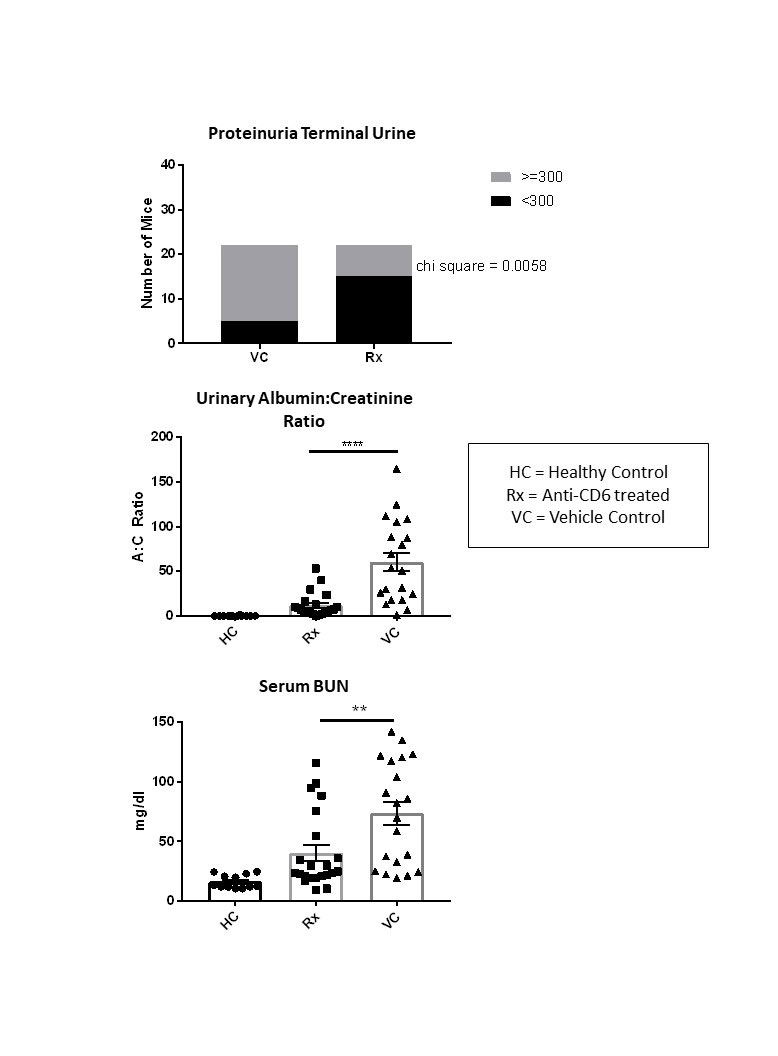
Methods: Nephrotoxic serum nephritis (NTN) is a validated, short-term model of LN. Disease was induced in two separate cohorts of female 129/svJ mice, both aged to 10 weeks. Mice were immunized with rabbit IgG and CFA on day 0 to create mouse anti-rabbit antibodies, which then cross-reacted with nephrotoxic rabbit serum given on day 5, causing an antibody-mediated nephritis similar in pathology to LN. To assess the importance of the CD6/ALCAM pathway in LN pathogenesis, mice were treated 3x per week with an anti-CD6 monoclonal antibody (mAb) (60ug/dose, n=23), or with vehicle control (n=23). Healthy mice (immunized with rabbit IgG, but not given nephrotoxic serum) were also included as a control (n=12). We monitored the progress of kidney disease via proteinuria (uristix), urinary albumin:creatinine ratio, and serum blood urea nitrogen (BUN) to assess the effect of the anti-CD6 treatment on both cohorts. To assess the affect of treatment on immune cell infiltration, flow cytometry, RT-PCR, and immunofluorescent staining was completed at termination.
Results: Mice treated with the anti-CD6 mAb displayed decreased levels of proteinuria (p< 0.001) compared to vehicle control mice. This result was confirmed by measuring albumin:creatinine ratios in terminal urine (p< 0.0001). We also found a significantly improved BUN (p< 0.01) when comparing treated mice to vehicle control mice. To ensure that anti-CD6 treatment did not interfere with the induction of the NTN model, we measured mouse anti-rabbit IgG levels and rabbit anti-mouse glomerular basement membrane (GBM) levels and found no difference between the groups. RT-PCR revealed significantly decreased levels of VCAM and RANTES in the kidneys of treated mice, while anti-inflammatory IL-10 was increased, compared to control sick mice. Flow cytometry results indicated decreased levels of renal-infiltrating activated T cells (CD4+CD25+CD69+, p < 0.01). Immunofluorescent staining and histology results are pending.
Conclusion: Inhibiting the CD6-ALCAM pathway with an anti-CD6 treatment is beneficial in ameliorating the nephritis associated with nephrotoxic antibody administration, an inducible model of lupus nephritis. These results indicate a potentially promising therapeutic option which is more selective than the immunosuppressive therapies currently offered.
To cite this abstract in AMA style:
Chalmers S, Garcia S, Ampudia J, Ng C, Connelly S, Putterman C. Amelioration of Immune Complex-Mediated Glomerulonephritis by CD6 Modulation [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/amelioration-of-immune-complex-mediated-glomerulonephritis-by-cd6-modulation/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/amelioration-of-immune-complex-mediated-glomerulonephritis-by-cd6-modulation/