Session Information
Date: Monday, November 18, 2024
Title: Systemic Sclerosis & Related Disorders – Basic Science Poster II
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Scleroderma (SSc), an autoimmune disease, features progressive fibrosis, leading to significant morbidity and mortality. Current therapies manage symptoms but lack efficacy in directly targeting or reversing fibrosis. Recent research implicates mechanotransduction pathways, such as the Rho/ROCK pathway, in SSc fibrosis. This pathway converges on non-muscle myosin II (NMII), a key regulator of cellular contractility. However, the specific roles of NMII isoforms (NMIIA and NMIIB) and their interaction with transforming growth factor beta (TGFβ), a well-established profibrotic mediator in SSc, remain unclear.
Methods: We investigated NMII pathway dysregulation in SSc using publicly available RNA microarray data (GSE58095; 61 SSc, 36 HD) and compared NMII activation in SSc vs healthy donor (HD) skin. Immunofluorescence and Western blotting assessed NMIIA, NMIIB distribution, and activation in HD and SSc fibroblasts ( n=8,7) primed or not with TGFβ . Rho activity was measured via pull-down assay. Pharmacological inhibitors targeting ROCK isoforms and NMII, alongside NMIIB knockdown with siRNA, explored the role of NMII in fibrosis. Production of profibrotic mediators (IL-6, collagen I) was quantified by ELISA and qPCR.
Results: Public data analysis revealed a significant upregulation of the Rho-ROCK-NMII pathway in SSc skin compared to HD (adjusted p < 0.0001). Consistent with this, our preliminary data showed a trend towards elevated MLC phosphorylation, a marker of NMII activation, in SSc skin biopsies compared to HD. SSc fibroblasts displayed a distinct redistribution of NMII isoforms with increased NMIIB localization towards stress fibers (p < 0.05), structures associated with fibrosis. Interestingly, TGFβ treatment mimicked this effect in HD fibroblasts (p < 0.01), suggesting a role for TGFβ in driving NMII isoform redistribution. Biochemical analysis confirmed these findings. Further investigation revealed a critical role for the Rho-ROCK-NMII pathway in TGFβ-induced fibrosis. Inhibiting ROCK or NMII activity significantly reduced TGFβ-mediated production of IL-6 and collagen I (p < 0.05) in both HD and SSc fibroblasts. Notably, ROCK2 inhibition displayed a specific effect on collagen production (p < 0.05), suggesting a potential therapeutic target. Importantly, knockdown of NMIIB, specifically, effectively countered the profibrotic effects of TGFβ, leading to decreased collagen and IL-6 release (p < 0.05). Interestingly, collagen inhibition occurred only at the protein level, while IL-6 inhibition was observed at both transcriptional and protein levels.
Conclusion: This study demonstrates dysregulation of the Rho-ROCK-NMII pathway, particularly involving NMIIB, in SSc skin fibrosis. Our findings suggest that targeting NMIIB or specific ROCK isoforms may hold promise for the development of antifibrotic therapies. Further investigation into the distinct roles of NMII isoforms and their regulation by TGFβ will provide valuable insights for refining therapeutic strategies in SSc.
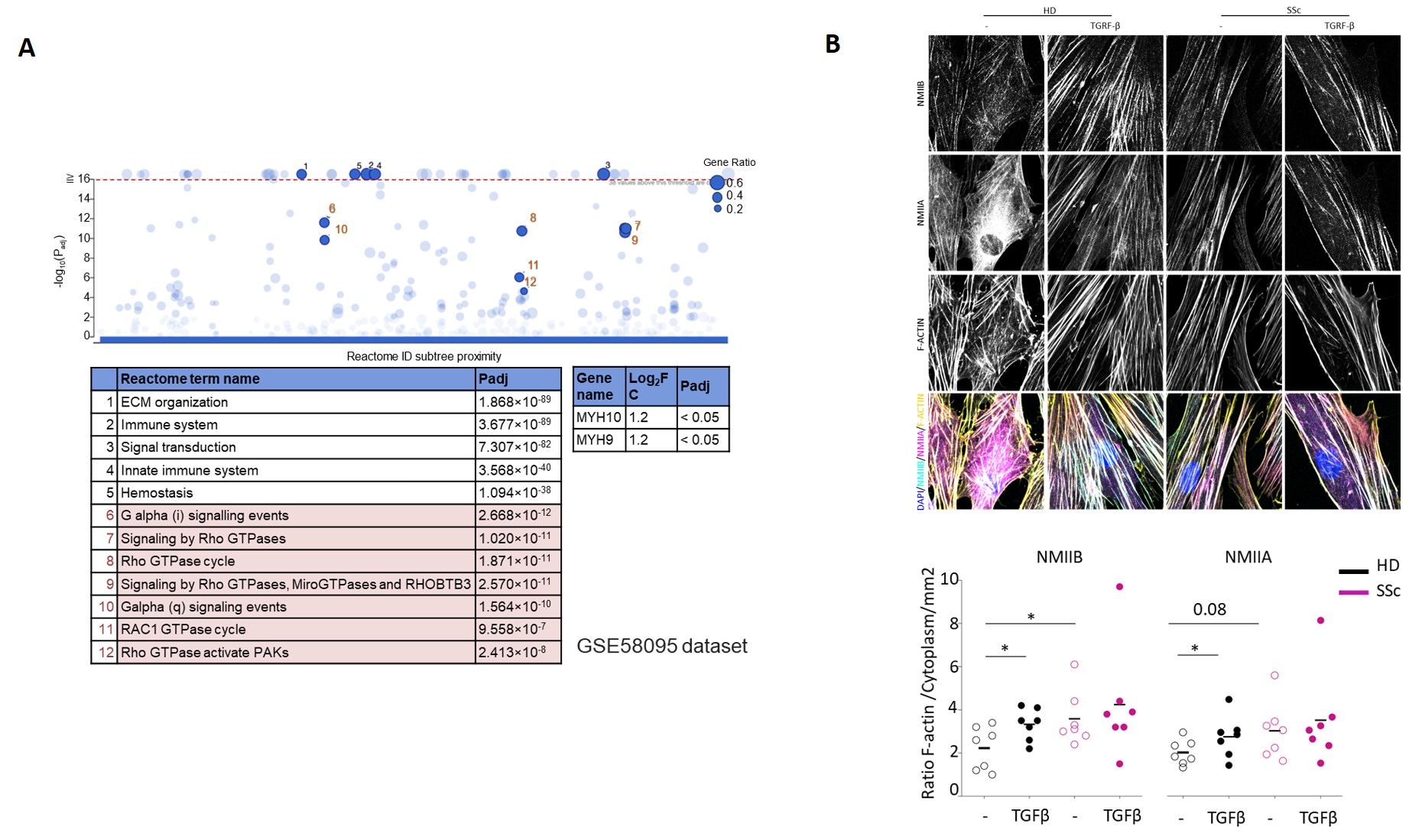
(A) RNA microarray analysis (GSE58095) reveals enrichment of Rho-ROCK-NMII pathway genes in SSc skin compared to healthy donors. (B) Immunofluorescence microscopy shows increased NMIIB (yellow) colocalization with F-actin stress fibers (magenta) in SSc fibroblasts compared to healthy donors (HD). Treatment with TGFβ further enhances this effect. Quantification of NMII isoform distribution in stress fibers confirms a significant increase in NMIIB but not NMIIA in SSc fibroblasts (n=7/group; *p<0.05). Scale bar: 10 µm.
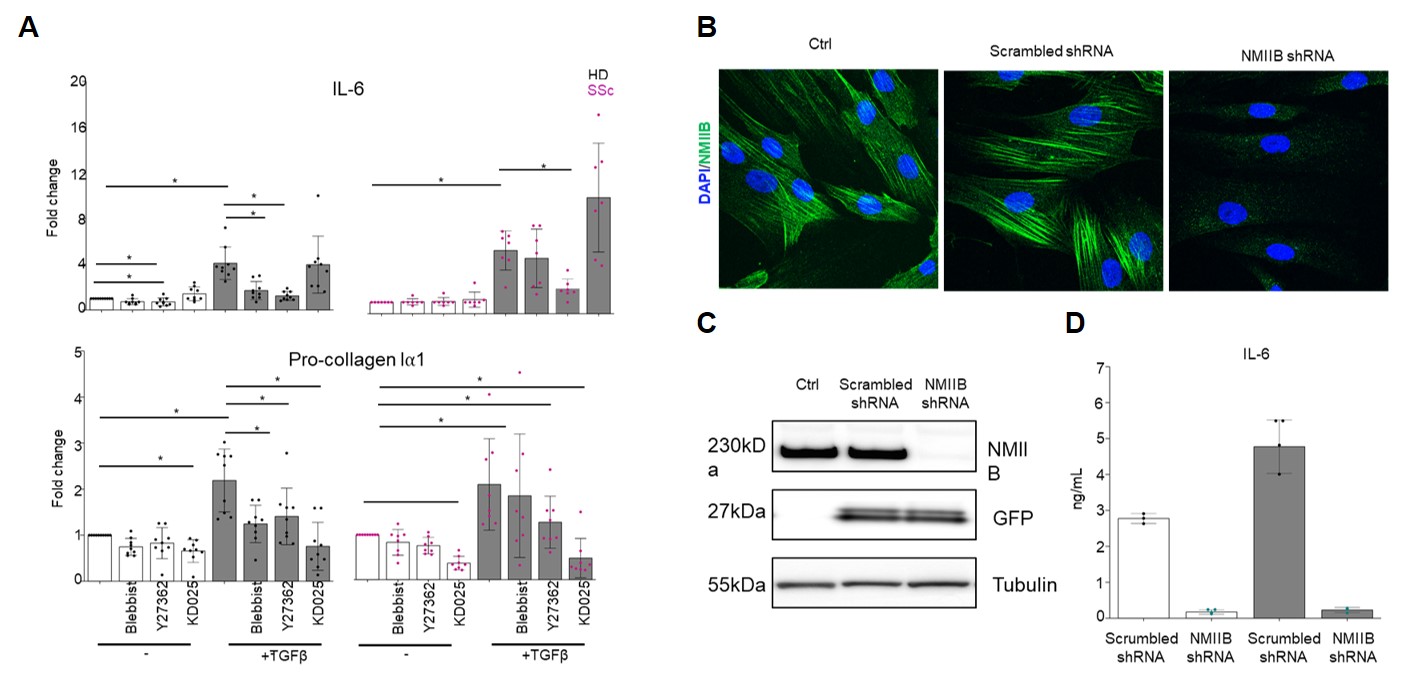
(A) Treatment with ROCK and NMII inhibitors significantly reduces IL-6 and collagen I production in both HD and SSc fibroblasts, both with and without TGFβ stimulation (n=8/8; *p<0.05). (B,C) Immunofluorescence (IF) and Western blot (WB) confirm efficient knockdown of NMIIB with siRNA . (D) Knockdown of NMIIB specifically reduces IL-6 production in HD fibroblasts, both with and without TGFβ stimulation ( representative experiment from three independent replicates.)
To cite this abstract in AMA style:
Russo B, Maria S, Noulet F, Romanescu G, brembilla N, Boehncke W. Altered Mechanotransduction via Myosin II Contributes to Collagen and IL-6 Production in Systemic Sclerosis Skin [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/altered-mechanotransduction-via-myosin-ii-contributes-to-collagen-and-il-6-production-in-systemic-sclerosis-skin/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/altered-mechanotransduction-via-myosin-ii-contributes-to-collagen-and-il-6-production-in-systemic-sclerosis-skin/