Session Information
Date: Sunday, November 17, 2024
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Although Takayasu’s arteritis (TAK) is not a prototypical autoantibody-mediated autoimmune disease, several lines of evidence suggested the pathogenic potential of B cells. However, limited data on the pathway of B cell activation and its underlying pathogenic role in TAK was reported.
Methods: Histology of paravascular lymph nodes from patients with TAK was studied. Bulk RNA-Seq, single-cell RNA-seq (scRNA-Seq) and flow cytometry were conducted to investigate the transcriptomic features and compositional characterizations of circulating B cells in TAK. The phenotype and function of age-associated B cells (ABCs) were elucidated by scRNA‐seq analysis, scATAC-seq analysis, flow cytometry, and in vitro experiments. The numeric and phenotypic changes of ABCs induced by pharmacological intervention were revealed by in vitro study and follow-up in patients with TAK.
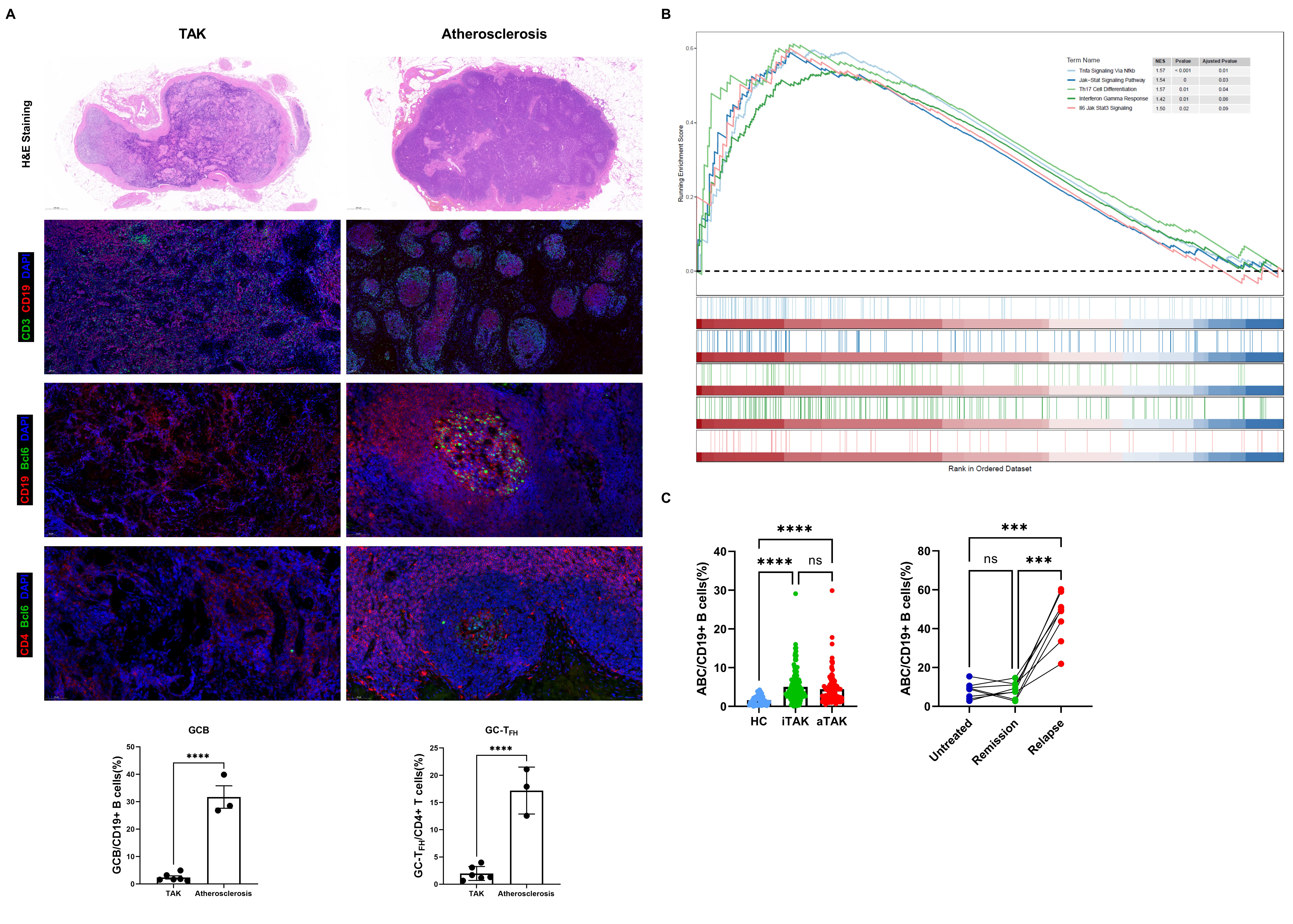
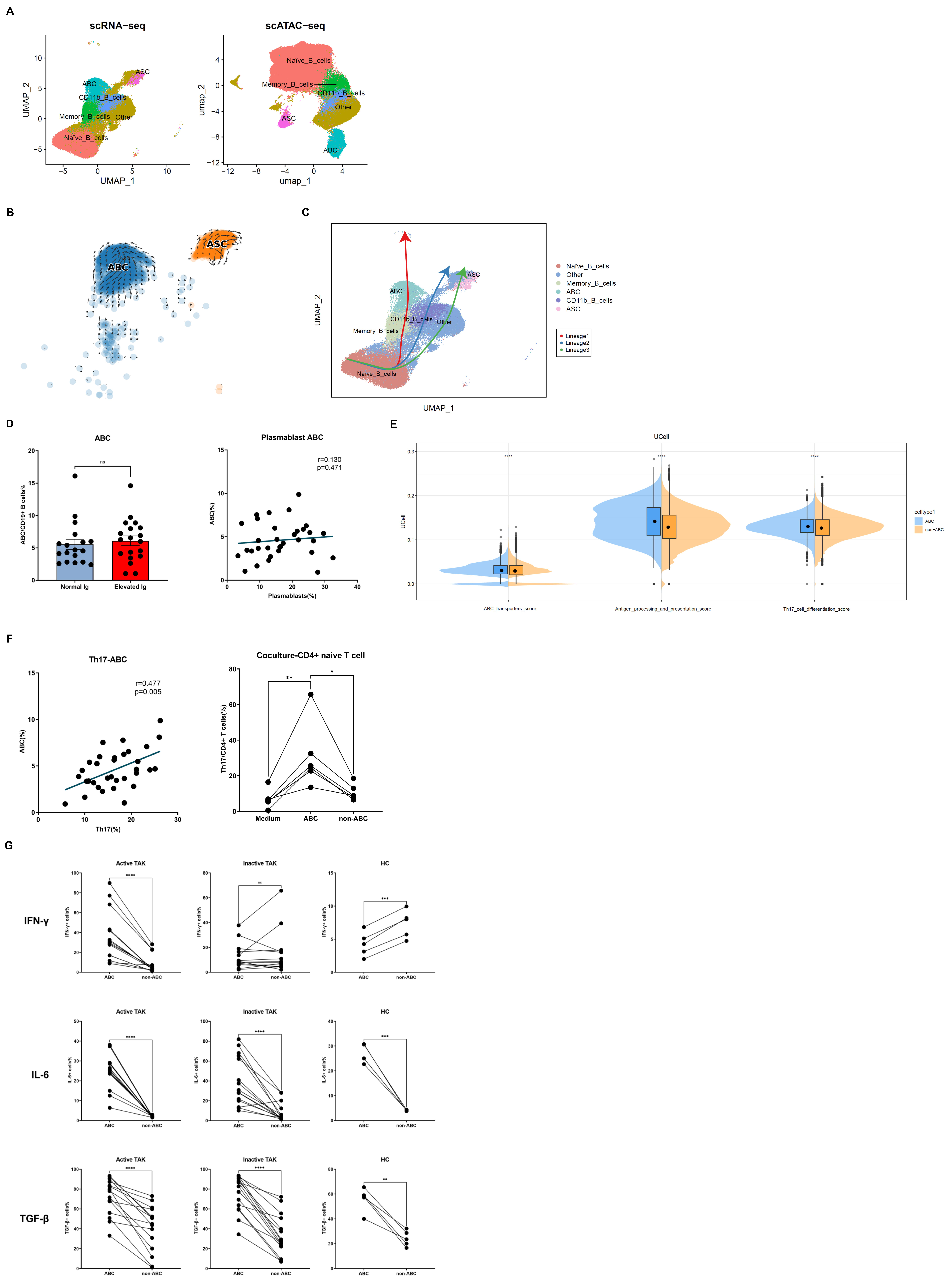
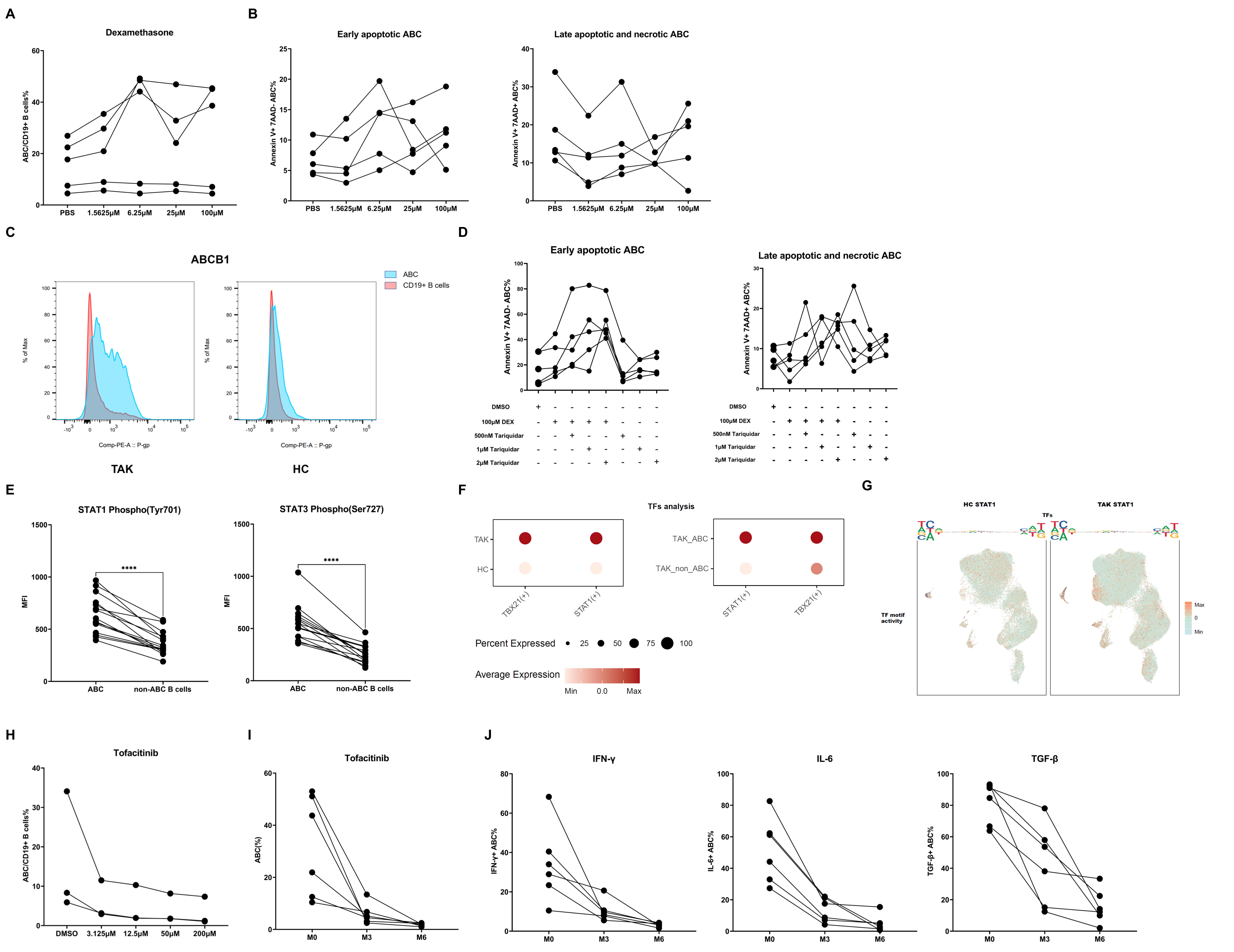
Results: Histological findings of paravascular lymph nodes suggested seriously disordered germinal center (GC), disrupted T- and B-cell zones, and marked reduction of germinal center type follicular T helper cells (GC-TFH) and GC B cells (GCB) (Figure 1). Upregulation of inflammation-linked pathways that were associated with extrafollicular (EF) response in circulating B cells from patients with TAK was revealed by bulk transcriptomic analysis (Figure 1). Flow cytometry showed the significantly increased proportions of circulating ABCs in TAK. Although the proportions of circulating ABCs did not correlate with disease activity, the association between ABCs and disease relapse was observed (Figure 1). Functionally, RNA velocity and Slingshot pseudotime analysis indicated that ABCs of patients with TAK had a completely different trajectory of differentiation from that towards antibody-secreting cells (ASCs). In contrast, ABCs exhibited distinct proinflammatory capability by intrinsically increasing inflammatory cytokines production and promoting Th17 response in TAK (Figure 2). Therapeutically, ABCs of patients with TAK have the property of glucocorticoid resistance mediated by ATP-binding cassette subfamily B member 1 (ABCB1). Upregulated STAT1 phosphorylation at Tyr701 and STAT3 phosphorylation at Ser727 were detected in ABCs of TAK, compared with non-ABC B cells counterpart. Transcription factor (TF) analysis and motif analysis showed that STAT1 was a critical TF in ABCs of TAK. In vitro experiments and clinical observation suggested that JAK inhibition led to both quantitative reduction and reversal of proinflammatory phenotype of ABCs in TAK (Figure 3).
Conclusion: Enhanced EF response and ABCs production are two prominent features of humoral immunity dysfunction in TAK. ABCs may contribute to the pathogenesis of TAK via the proinflammatory phenotype in distinct manners independent of ASCs differentiation. JAK inhibition is a promising therapy that suppresses ABCs in TAK.
To cite this abstract in AMA style:
Fang C, Sun X, Jin S, du L, Li J, Zeng X, Chen Y, Li M, Tian X. Age-associated B Cells Contribute to Inflammation of Takayasu’s Arteritis in an Antibody-secreting Cell Differentiation Independent Manner [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/age-associated-b-cells-contribute-to-inflammation-of-takayasus-arteritis-in-an-antibody-secreting-cell-differentiation-independent-manner/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/age-associated-b-cells-contribute-to-inflammation-of-takayasus-arteritis-in-an-antibody-secreting-cell-differentiation-independent-manner/