Session Information
Date: Monday, November 8, 2021
Title: RA – Treatments Poster II: PROs, Biomarkers, & Systemic Inflammation (1223–1256)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Previous studies have found differential treatment efficacy of abatacept (ABA) for the treatment of RA based on biomarker-seropositivity.1-4 An earlier study found a differential treatment impact of ABA among SeroPositive Early & Active RA (SPEAR) patients in historic randomized controlled trials (RCTs).5 In this study, we add to this evidence and test the robustness of the differential treatment impact of ABA among patients with SPEAR to adjustment for patient characteristics.
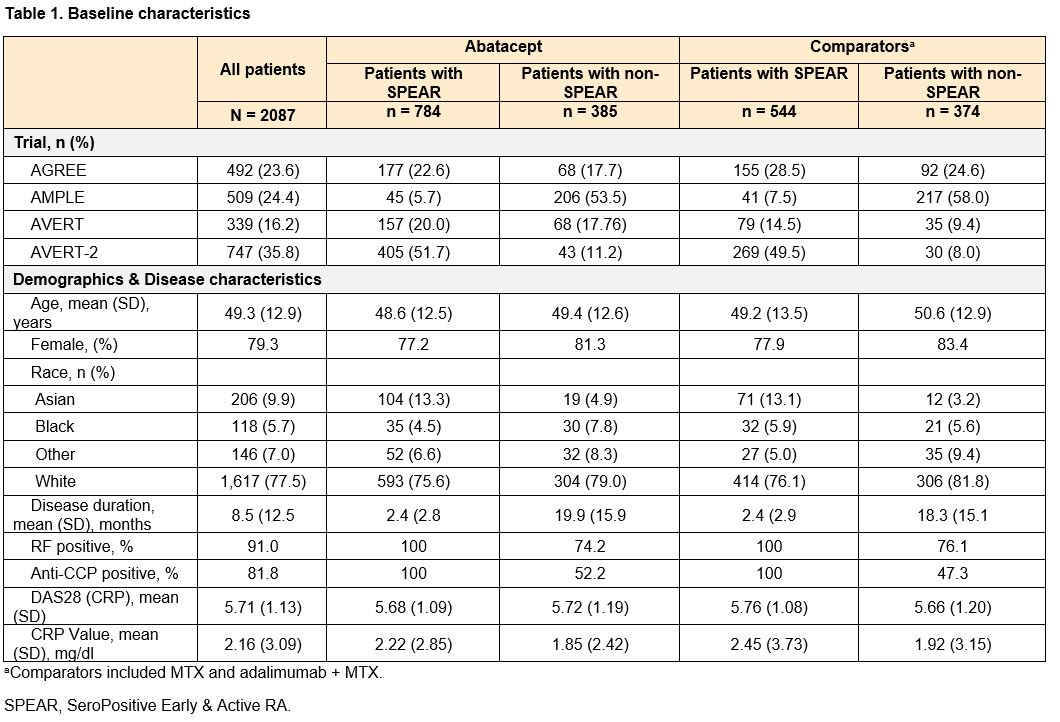
Methods: Pooled patient data from 2087 patients from 4 phase 3, early-RA ABA RCTs (AGREE [NCT00122382], AMPLE [NCT00929864], AVERT [NCT01142726], AVERT-2 [NCT02504268]) were analyzed. Patients were defined as SPEAR if they satisfied the following baseline criteria: 1) RF+ and > 3 times the upper limit of normal on an anti-CCP test (ACPA+), 2) disease duration < 12 months, and 3) DAS28 (CRP) > 3.2. Outcomes included DAS28-CRP and SDAI mean change from baseline to week 24 and remission (< 2.6 or <3.3, respectively), and ACR 20/50/70 at week 24. Patients were treated with either ABA (monotherapy or ABA+MTX) or a comparator (MTX or adalimumab + MTX). Analyses were conducted separately among ABA-treated patients (comparing SPEAR vs non-SPEAR) and the full population (estimating how the efficacy of ABA vs comparators is modified by SPEAR status). For each outcome, two separate analyses were conducted: the base model included trial fixed effects, SPEAR status, baseline measures of the outcome (for DAS28-CRP or SDAI outcomes), and treatment type and an interaction between treatment type and SPEAR status (when analyzing the full population). In a further adjusted model, baseline demographics (age, gender, race (White, Non-White), region (North America, Europe, Other)) and baseline ACR core measures were added as covariates. Sensitivity analyses defining SPEAR status using only ACPA+ and RF+ were conducted.
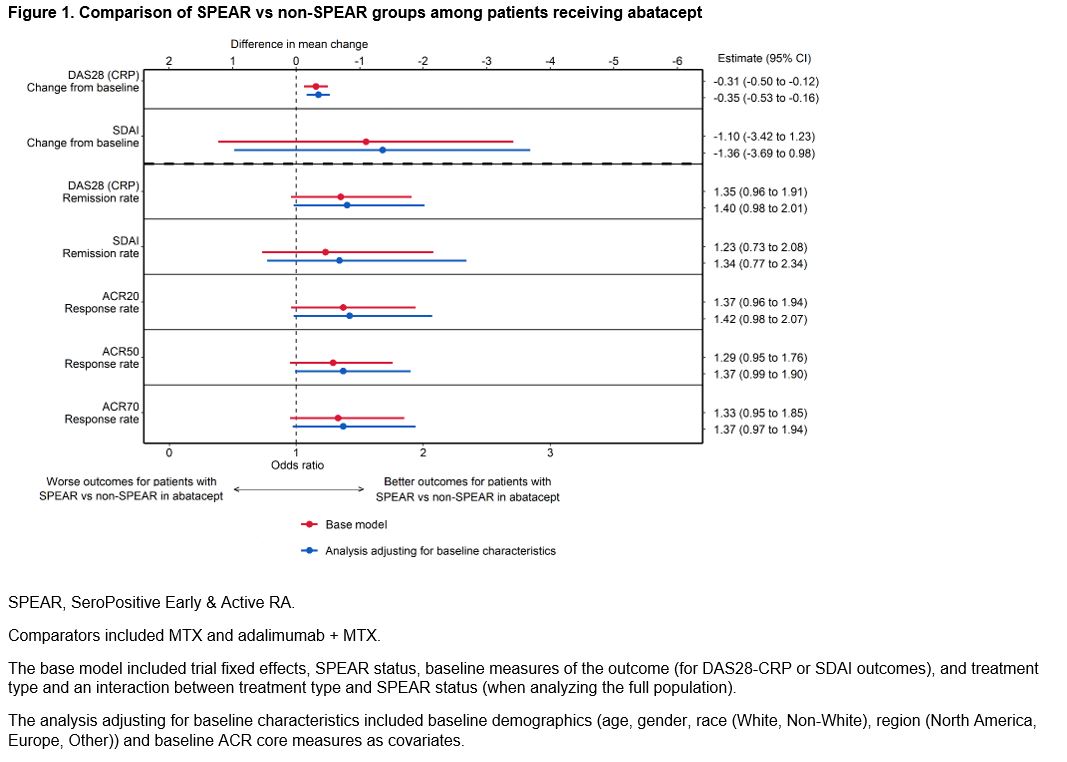
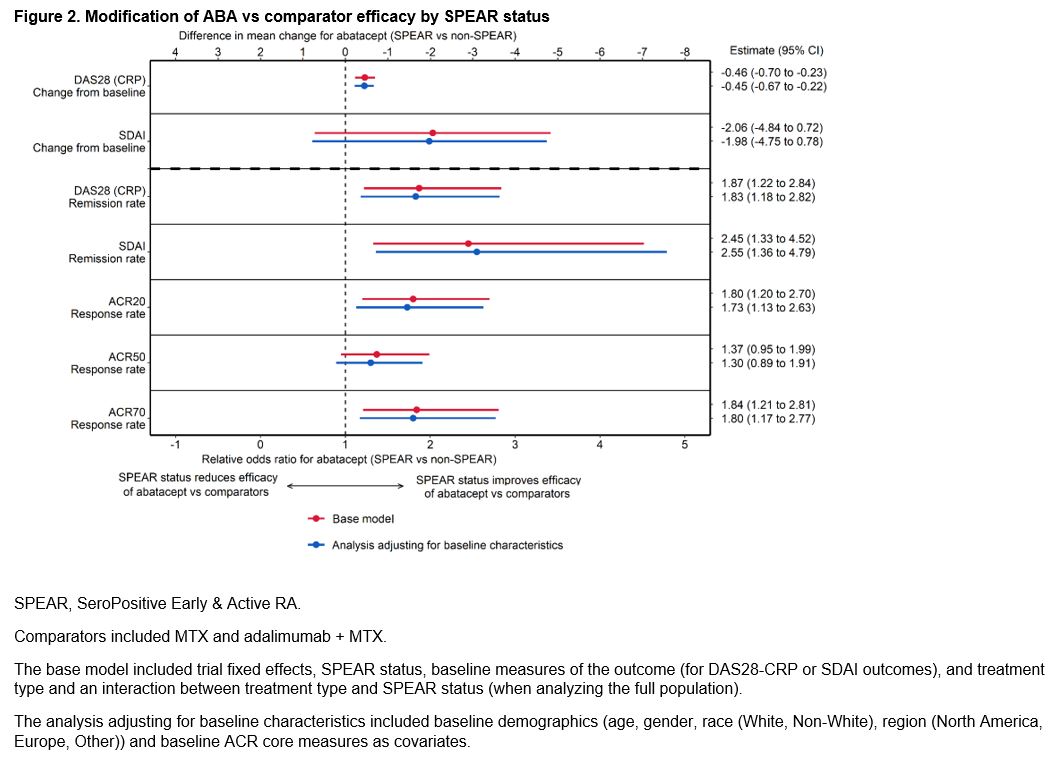
Results: This study analyzed 1328 (64%) SPEAR and 759 (36%) non-SPEAR patients. Roughly half of patients with non-SPEAR were RF+ and three-quarters ACPA+; while all patients had high DAS28 (CRP) (Table 1). The estimated differential treatment efficacy of ABA among SPEAR patients for both analyses – improved outcomes for ABA-treated SPEAR patients vs non-SPEAR patients, and improved relative efficacy for ABA vs comparators among SPEAR patients vs non-SPEAR patients – was robust to including additional baseline characteristics across all outcomes, with only small changes to effect sizes and precision (Figures 1 & 2). Findings were consistent in sensitivity analyses.
Conclusion: This analysis compared clinical outcomes of RA patients with enriched autoantibody biomarkers and early disease stage (SPEAR) to non-SPEAR, and found that the differential efficacy of ABA was robust to adjustment for baseline demographics and disease status.
References:
1. Buckner J, et al. Arthritis Rheumatol 2019;71(Suppl 10):abstract 1424.
2. Huizinga T, et al. Ann Rheum Dis 2015;74:234–235.
3. Harrold LR, et al. Rheumatol Ther 2019;6:217–230.
4. Sokolove J, et al. Ann Rheum Dis 2016;75:709–714.
5. Michaud K, et al. Ann Rheum Dis POS0474, in press.
To cite this abstract in AMA style:
Pope J, Park S, Fillbrunn M, Lozenski K, Khaychuk V, Michaud K, Signorovitch J, Lane H, Nguyen H, Conaghan P. Adjusted Analyses of the Benefits of Autoantibody Enrichment on Efficacy Outcomes in Early RA, from a Pooled Analysis of 4 Abatacept RCTs [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/adjusted-analyses-of-the-benefits-of-autoantibody-enrichment-on-efficacy-outcomes-in-early-ra-from-a-pooled-analysis-of-4-abatacept-rcts/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/adjusted-analyses-of-the-benefits-of-autoantibody-enrichment-on-efficacy-outcomes-in-early-ra-from-a-pooled-analysis-of-4-abatacept-rcts/