Session Information
Session Type: Poster Session
Session Time: 10:30AM-12:30PM
Background/Purpose: Knee osteoarthritis (KOA) is a leading cause of disability, with no available disease-modifying treatments. While Hyaluronic Acid (HA) remains widely used, there is little to no head-to-head randomised evidence comparing HA with adipose-derived mesenchymal stem cells (AdMSC) therapy. This evaluator-blinded, phase IIb trial compared the efficacy, safety and dose response of autologous adipose-derived mesenchymal stem cells (AdMSC) versus intermediate molecular-weight HA in patients with refractory primary KOA in Bangladesh.
Methods: Seventy-three participants with Kellgren-Lawrence grade ≥2 KOA on standard conservative care were randomised to receive HA injection (n=31), a single-dose AdMSC injection (n=21), or two-dose AdMSC injections (n=21, dose interval 3 months). Primary outcomes included pain on VAS, knee-related problems on KOOS, femoral cartilage thickness utilising ultrasound scan, and MRI-assessed cartilage defects (MOAKS) at baseline, 6, and 12 months involving a multidisciplinary clinical team.
Results: Interim analysis revealed significant, dose-dependent improvements. Two-dose AdMSC resulted in greater pain reduction (3.2 vs. 1.3 points) and functional improvement (21.24 vs. 11.31 points) compared to HA at 6 months (p < 0.001). Cartilage thickness increased (0.25±0.18mm) and defects improved (9.13%) with two-dose AdMSC, whereas the HA group showed deterioration (-0.06±0.14mm; 0.75%). Single-dose AdMSC also showed better outcomes than HA. No baseline predictors were identified, and all treatments were well-tolerated.
Conclusion: Two-dose AdMSC therapy significantly outperformed HA and single-dose AdMSC in improving pain, function, and cartilage structure, indicating its potential as a disease-modifying treatment for KOA. These results support advancing to larger phase III trials, particularly in South Asia, where KOA is a growing health burden. (www.clinicaltrials.gov: NCT05783154, IRB approval reference: BSMMU/2024/1192/registration/742).
.jpg) Table 1: Summary of the baseline clinical characteristics of the study population (Nf73). SD, standard deviation, VAS, visual analogue scale (0-10; 0 indicates no pain and 10 indicates worst pain), KOOS, The Knee Injury and Osteoarthritis Outcome Score (0-100 with 0 representing extreme knee problems and 100 representing no knee problems).
Table 1: Summary of the baseline clinical characteristics of the study population (Nf73). SD, standard deviation, VAS, visual analogue scale (0-10; 0 indicates no pain and 10 indicates worst pain), KOOS, The Knee Injury and Osteoarthritis Outcome Score (0-100 with 0 representing extreme knee problems and 100 representing no knee problems).
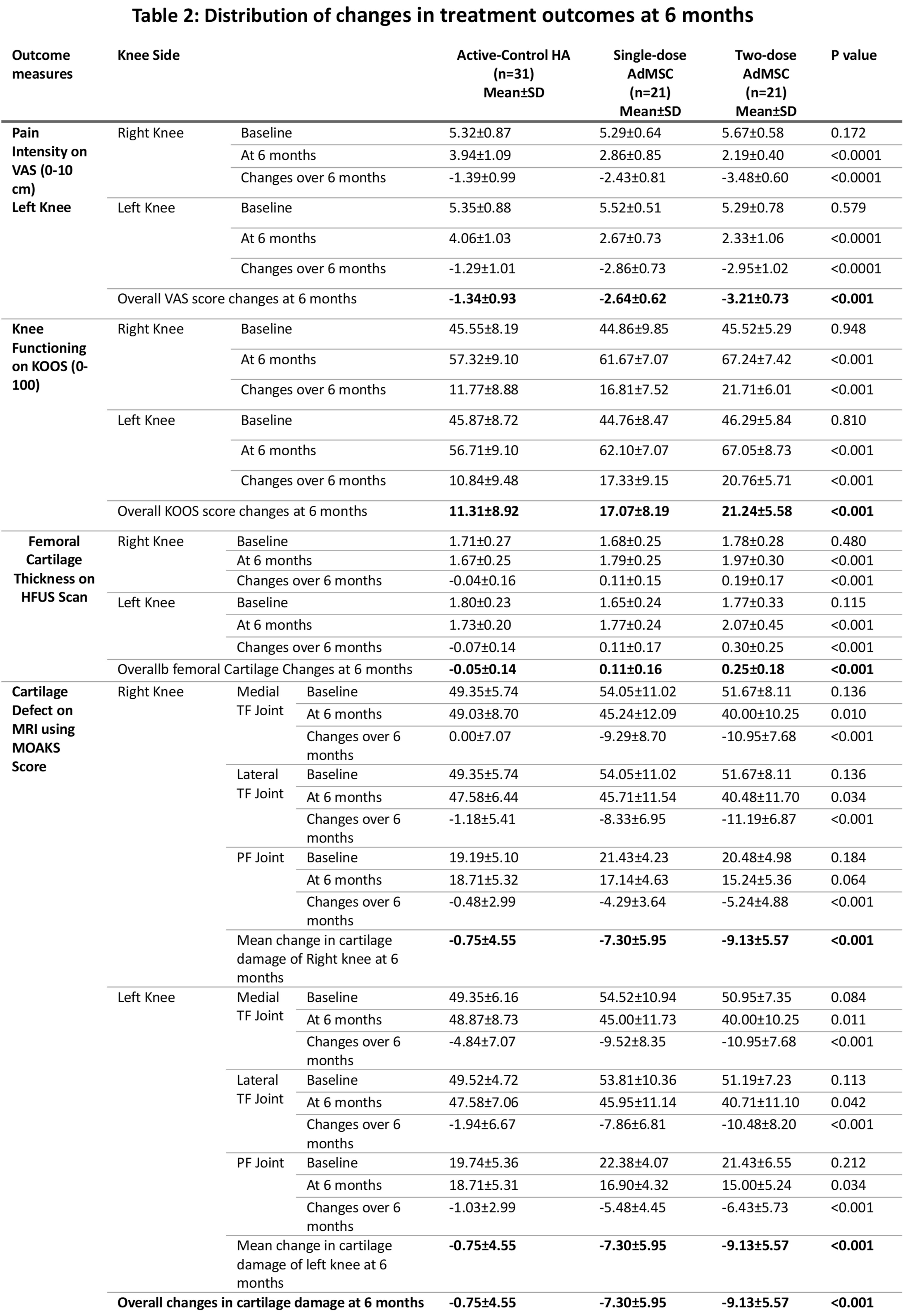 Changes in pain on VAS, knee damage on KOOS, femoral cartilage thickness on ultrasound, and cartilage defect on MOAKS at 6 months
Changes in pain on VAS, knee damage on KOOS, femoral cartilage thickness on ultrasound, and cartilage defect on MOAKS at 6 months
.jpg) Figure 1: Forest plot for changes in articular cartilage defect in the knee of single-dose AdMSC and two-dose AdMSC compared to HA. Changes in femoral cartilage thickness of a two-dose AdMSC recipient at 6 months.
Figure 1: Forest plot for changes in articular cartilage defect in the knee of single-dose AdMSC and two-dose AdMSC compared to HA. Changes in femoral cartilage thickness of a two-dose AdMSC recipient at 6 months.
To cite this abstract in AMA style:
Khasru M, Islam M, Jubery A, Shirin M, Chowdhury F, Marzen T, Uzzaman N, Siddiq M, Hoque M, Begum M, Khan M, Salek A. Adipose-Tissue Derived Mesenchymal Stem Cells vs Hyaluronic Acid in Refractory Knee Osteoarthritis in a low-resource setting: A Phase IIb RCT [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/adipose-tissue-derived-mesenchymal-stem-cells-vs-hyaluronic-acid-in-refractory-knee-osteoarthritis-in-a-low-resource-setting-a-phase-iib-rct/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/adipose-tissue-derived-mesenchymal-stem-cells-vs-hyaluronic-acid-in-refractory-knee-osteoarthritis-in-a-low-resource-setting-a-phase-iib-rct/
