Session Information
Date: Sunday, November 12, 2023
Title: (0066–0095) T Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: In humans, upper respiratory infections have been associated with onset of Rheumatoid Arthritis. In mice, following viral infection, CD8+ T cells take up residence in joints and a portion become tissue resident memory cells (TRM). As these CD8+ TRM are not specific for a Rheumatoid Arthritis specific self-peptide, it is not clear if they contribute to disease activity. In the mouse uterus, re-activation of CD8+ TRM increases the permeability of the tissue to circulating antibodies. Utilizing the K/BxN serum transfer model, we demonstrate that local activation of CD8+ TRM generates persistent clinical arthritis at that site.
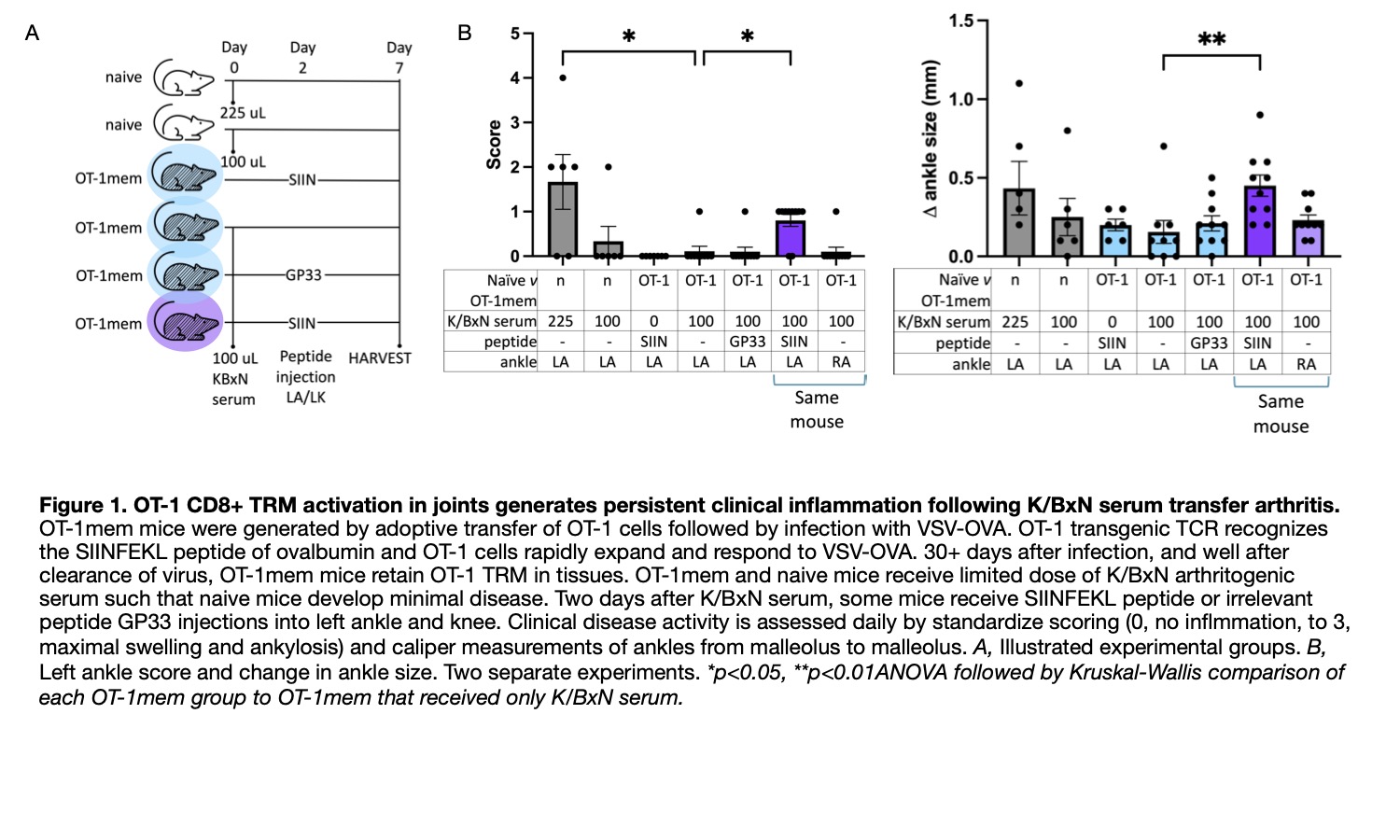
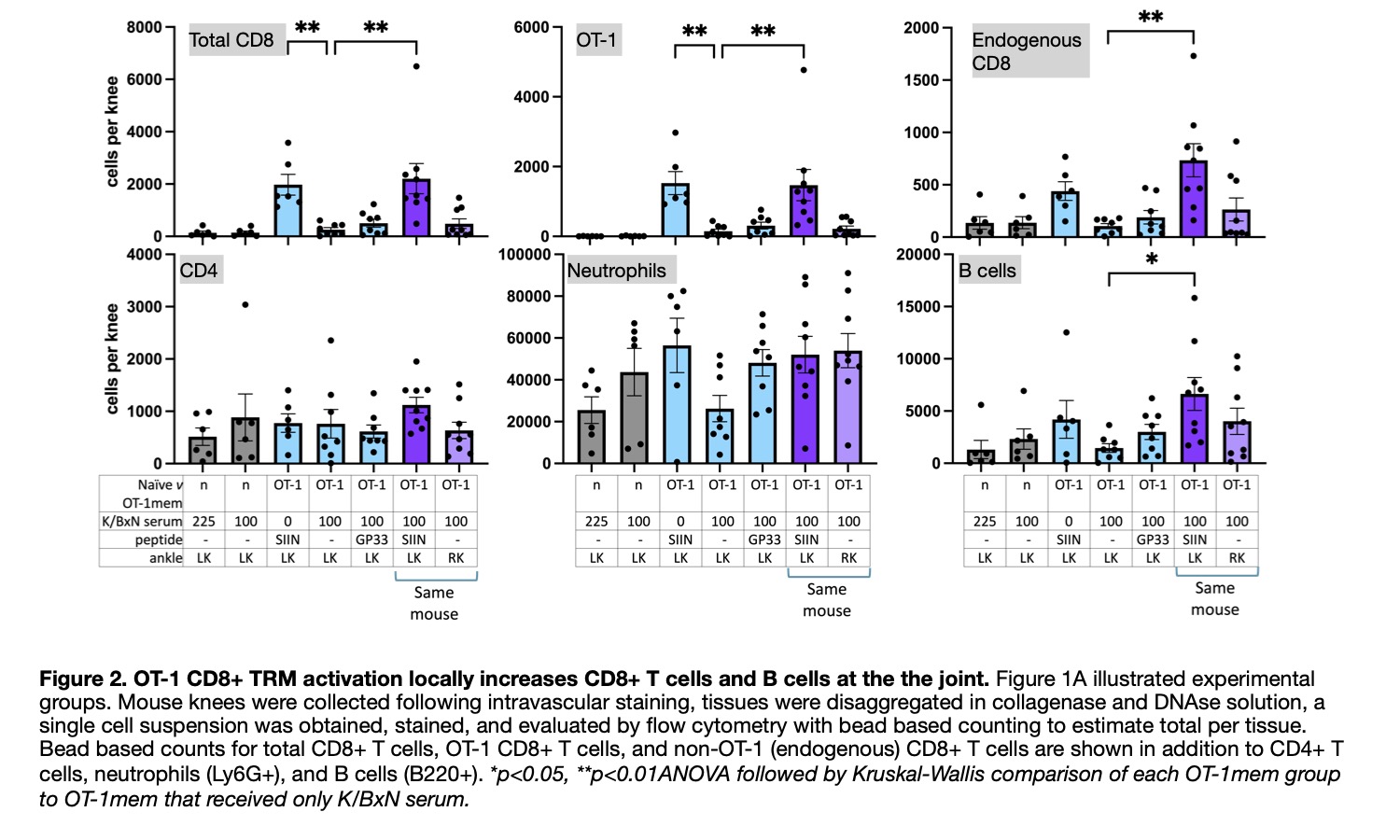
Methods: C57BL/6 mice received adoptive transfer of OT-1 transgenic CD8+ T cells, whose cognate antigen is SIINFEKL peptide. The next day, they are infected with vesicular stomatitis virus encoding SIINFEKL. Acute infection resolves over the course of a few weeks, virus is cleared, but tissues are persistently populated by OT-1 CD8+ TRM. These mice are OT-1mem mice. Naïve and OT-1mem mice receive dose limited volumes of arthritogenic serum from K/BxN mice. Two days later, they receive an injection of SIINFEKL peptide in their left ankle and left knee to reactivate OT-1 TRM. Ankles were scored for severity of clinical inflammation (0, no swelling/redness; 1, mild swelling/redness +/- swollen digits; 2, moderate swelling/redness; and 3, maximal swelling/redness with ankylosis) and their size was measured by calipers daily (from lateral to medial malleolus). After seven days, mice were sacrificed following intravascular stain injection, ankles fixed and decalcified for histology, and knees collected and processed to a single cell suspension for flow cytometry. (See Figure 1A for illustration of experimental groups.)
Results: Persistent clinical inflammation and increased size was found in the left ankle of OT-1mem mice that received K/BxN serum and injection of SIINFEKL, but not their right ankle, not in the left ankle of memory mice injected with an irrelevant peptide GP33, and not in the left ankle of memory mice that received SIINFEKL. (Figure 1B) By flow cytometry, the left, but not right, knee of memory mice that received K/BxN serum and injection of SIINFEKL contained significantly more OT-1 cells, endogenous CD8+ T cells, and B220+ B cells compared to Ot-1mem mice that received only K/BxN serum. This was not true of the left knee of OT-1mem mice that received K/BxN serum and left knee injection with GP33, nor the left knee of OT-1mem mice that received only SIINFEKL injection. (Figure 2)
Conclusion: Persistent clinical inflammation is dependent on both K/BxN serum and local activation of virus specific joint CD8+ TRM and characterized by increased number of OT-1 transgenic CD8+ T cells, endogenous CD8+ T cells, and B220+ B cells. Thus, ‘bystander,’ virus specific, CD8+ TRM activation can localize systemic autoantibody mediated processes to the joint.
To cite this abstract in AMA style:
Lotfi-Emran S, Koziol N, Masopust D. Activation of Joint CD8+ TRM Localize Autoantibody Mediated Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/activation-of-joint-cd8-trm-localize-autoantibody-mediated-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/activation-of-joint-cd8-trm-localize-autoantibody-mediated-arthritis/