Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Gain-of-function mutations in STING, a critical mediator of dsDNA sensing, lead to a severe autoinflammatory syndrome known as STING-Associated Vasculopathy with onset in Infancy (SAVI). SAVI patients develop interstitial lung disease (ILD) and produce autoantibodies that are commonly associated with systemic autoimmunity. While mice expressing the most common SAVI mutation STING V154M (VM) similarly develop ILD, they exhibit severe lymphopenia, low serum immunoglobulin (Ig) titers, and fail to develop autoantibodies. This difference likely arises from differential expression of STING in human versus mouse B cells, as STING activation promotes cell death in murine B cells, whereas human B cells express minimal STING. Importantly, lethally irradiated VM hosts reconstituted with wildtype (WT) stem cells (WT→VM) still develop ILD, indicating that hematopoietic VM expression is not required for disease. Here, we investigate whether expression of the VM mutation in radioresistant cells is sufficient to recapitulate the development of autoimmunity seen in SAVI patients.
Methods: To demonstrate whether WT→VM mice show unimpaired B cell function, we assessed serum Ig titers and performed B cell proliferation assays in VM and WT→VM mice alongside littermate controls. To determine if lymphocyte repertoire contributed to lung-resident lymphocyte recruitment and activation in mice expressing the VM mutant, we generated mixed bone marrow (BM) chimeras using transgenic (Tg) BCR (MD4) and Tg TCR (OTI/OTII) BM in competition with WT BM and compared the activation of Tg and WT donor derived lymphocytes in lung tissues by flow cytometry. Lastly, we evaluated the presence of lymphocyte autoreactivity using anti-nuclear antibody assays, auto-antigen arrays, and western blot analysis with sera from WT→VM mice, as well as assessing for the development of lung disease following adoptive transfer of splenocytes from WT→VM chimeric mice to naïve Rag1-/- hosts.
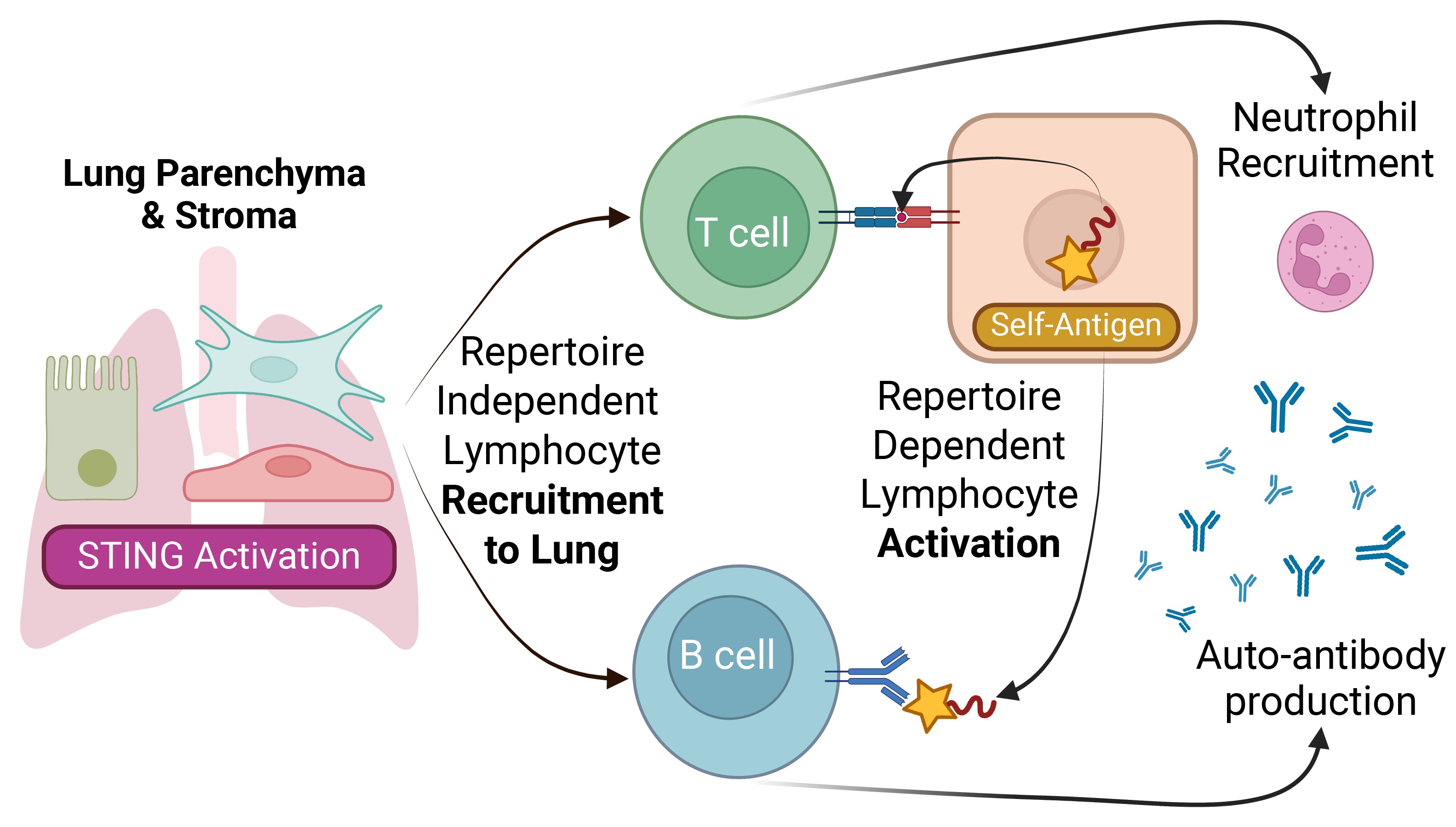
Results: We now show WT→VM chimeras have restored B cell function, form germinal centers within lung tissues, produce autoantibodies, and thereby recapitulate the loss of tolerance seen in SAVI patients. Lymphocytes derived from both WT and BCR or TCR Tg donors accumulate in lung tissues of WT+TgàVM mixed chimeras, but lymphocyte activation and germinal center formation require WT cells with a diverse repertoire. Furthermore, when splenic T cells from the WTàVM chimeras were adoptively transferred to naïve Rag1-/- secondary hosts, they trafficked to the lung and recruited neutrophils.
Conclusion: Collectively, our data supports a multi-step process wherein STING activation within radioresistant cells promotes a repertoire-independent recruitment of immune cells into lung tissues, and subsequent repertoire-dependent activation of B and T cells results in the development of humoral and cell-mediated autoimmunity against both lung and systemic self-antigens. Our findings provide insight into how STING activation within stromal and parenchymal tissues initiates tolerance-break and highlights the need to further investigate how cGAS-STING and other innate immune sensing pathways within non-hematopoietic cells contribute to autoimmune pathogenesis.
To cite this abstract in AMA style:
Gao K, Chiang K, Subramanian S, Yin X, Utz P, Nundel K, Fitzgerald K, Marshak-Rothstein A. Activation of Autoreactive Lymphocytes in the Lung by STING Gain-of-function Mutation Expressing Radioresistant Cells [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/activation-of-autoreactive-lymphocytes-in-the-lung-by-sting-gain-of-function-mutation-expressing-radioresistant-cells/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/activation-of-autoreactive-lymphocytes-in-the-lung-by-sting-gain-of-function-mutation-expressing-radioresistant-cells/