Session Information
Date: Saturday, November 12, 2022
Title: Spondyloarthritis Including PsA – Treatment Poster I: AxSpA
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
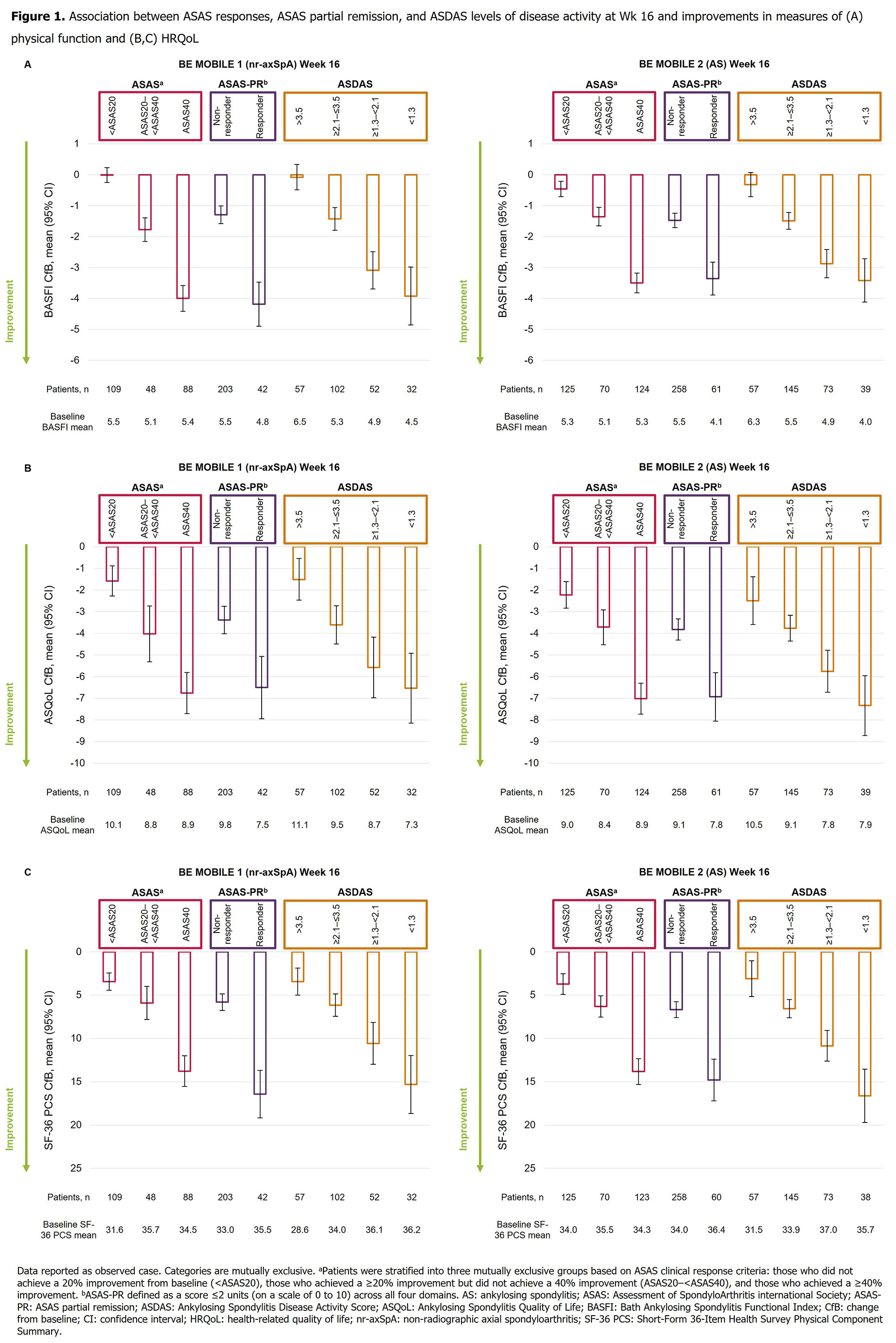
Background/Purpose: Examine the association between achieving increasingly stringent clinical response criteria and lower levels of disease activity, and improvements in physical function and health-related quality of life (HRQoL), in patients with axial spondyloarthritis (axSpA).
Methods: This post hoc analysis reports pooled (placebo/bimekizumab 160 mg Q4W) Week (Wk) 16 results from the phase 3 studies, BE MOBILE 1 (NCT03928704; non-radiographic axSpA; [nr-axSpA]) and BE MOBILE 2 (NCT03928743; ankylosing spondylitis [AS]; i.e. radiographic axSpA).1,2 In BE MOBILE 1, patients met the Assessment of SpondyloArthritis international Society (ASAS) classification criteria and had elevated C-reactive protein and/or MRI sacroiliitis. In BE MOBILE 2, patients showed radiographic evidence of AS, fulfilling modified New York criteria. All patients who reached specified clinical response criteria (ASAS: 20% improvement from baseline not reached [< ASAS20], ≥20% reached but 40% not reached [ASAS20–< ASAS40], ≥40% reached [ASAS40]; ASAS partial remission [ASAS-PR]: Yes, No) or levels of disease activity (ASDAS: >3.5, ≥2.1–≤3.5, ≥1.3–< 2.1, < 1.3) at Wk 16 were pooled regardless of treatment arm, by study. Associations between achievement of these specified clinical response criteria/levels of disease activity and improvements in patient-reported measures of physical function (BASFI: 0 [best] to 10 [worst]) and HRQoL (ASQoL: scored from 0 [best] to 18 [worst]; SF-36 PCS: standardized measure, higher score reflects better HRQoL) were assessed; it should be noted that some aspects of these clinical response criteria relate to aspects of BASFI. Observed case data reported.
Results: The majority of patients completed Wk 16 (nr-axSpA: 244/254 [96.1%]; AS: 322/332 [97.0%]). Baseline characteristics between nr-axSpA and AS patients, including BASFI, ASQoL, and SF-36 PCS scores were comparable, indicating consistent physical function and HRQoL burden. Patients achieving higher ASAS response ranges demonstrated sequentially and significantly greater mean (95% CI) improvements from baseline in BASFI scores across studies (nr-axSpA: < ASAS20: 0.0 [−0.2, 0.2], ASAS20–< ASAS40: −1.8 [−2.2, −1.4], ASAS40: −4.0 [−4.4, −3.6]); AS: −0.5 [−0.7, −0.2], −1.4 [−1.7, −1.1], −3.5 [−3.8, −3.2]; Figure 1A). Achievement of higher ASAS response ranges was also associated with improvements in ASQoL scores (nr-axSpA: < ASAS20: −1.6 [−2.3, −0.9], ASAS20–< ASAS40: −4.0 [−5.3, −2.7], ASAS40: -6.8 [−7.7, −5.8]); AS: −2.2 [−2.8, −1.6], −3.7 [−4.5, −2.9], −7.0 [−7.7, −6.3]; Figure 1B); similar improvements were seen for SF-36 PCS (Figure 1C), and with ASAS-PR and ASDAS (Figure 1). In all cases, improvements in BASFI, ASQoL, and SF‑36 PCS were of similar amplitude between nr-axSpA and AS patients.
Conclusion: Patients with nr-axSpA and AS who achieved increasingly stringent clinical response criteria and lower levels of disease activity at Wk 16 reported significantly greater improvements in physical function and HRQoL, irrespective of treatment.
References: 1. Deodhar A. Ann Rheum Dis 2022;81:772─3; 2. van der Heijde D. Ann Rheum Dis 2022;81:12─3.
To cite this abstract in AMA style:
Magrey M, Deodhar A, Mease P, Navarro-Compán V, Ramiro S, Rudwaleit M, de la Loge C, Fleurinck C, Taieb V, Mørup M, Oortgiesen M, Kay J. Achieving Increasingly Stringent Clinical Response Criteria & Lower Levels of Disease Activity Is Associated with Greater Improvements in Physical Function & HRQoL in Patients with Active Axial Spondyloarthritis: 16-Week Results from Two Phase 3 Randomized, Placebo-Controlled Studies [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/achieving-increasingly-stringent-clinical-response-criteria-lower-levels-of-disease-activity-is-associated-with-greater-improvements-in-physical-function-hrqol-in-patients-with-active-axial-spondy/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/achieving-increasingly-stringent-clinical-response-criteria-lower-levels-of-disease-activity-is-associated-with-greater-improvements-in-physical-function-hrqol-in-patients-with-active-axial-spondy/