Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: ASDAS-CRP is a composite score that measures disease activity in axial spondyloarthritis (axSpA) and is based on patient-reported outcomes and objective measures of inflammation. An ASDAS-CRP score of < 1.3 indicates inactive disease in axSpA clinical trials and is considered equivalent to clinical remission. We hypothesized that achieving ASDAS-CRP< 1.3 is not feasible in clinical practice. We aimed to evaluate the proportion of patients achieving inactive disease compared to low disease activity (ASDAS< 2.1) with axSpA.
Methods: Our PICO question was what proportion of patients with axSpA could reach inactive disease status compared to low disease activity in the treatment group in clinical trials with Biologics and targeted disease-modifying agents. A comprehensive literature search was conducted using electronic databases, including PubMed, Embase, and Cochrane Library, and screening the EU clinical trial registry to identify relevant studies published until May 2023. Clinical trials reporting inactive disease and low disease activity were included. The studies included patients with both radiographic and non-radiographic axSpA treated with different biologic therapies (TNF-i, IL-17-i, JAK-i). The risk of bias was assessed using the Cochrane risk of bias tool. Two independent reviewers screened the articles, and the senior author did conflict resolution. Data were extracted and analyzed using forest plot to compare odds ratios (95% CI). Study heterogeneity was assessed using I2. All analyses were performed using R Statistical Software meta package (v4.2.1; R Core Team 2022).
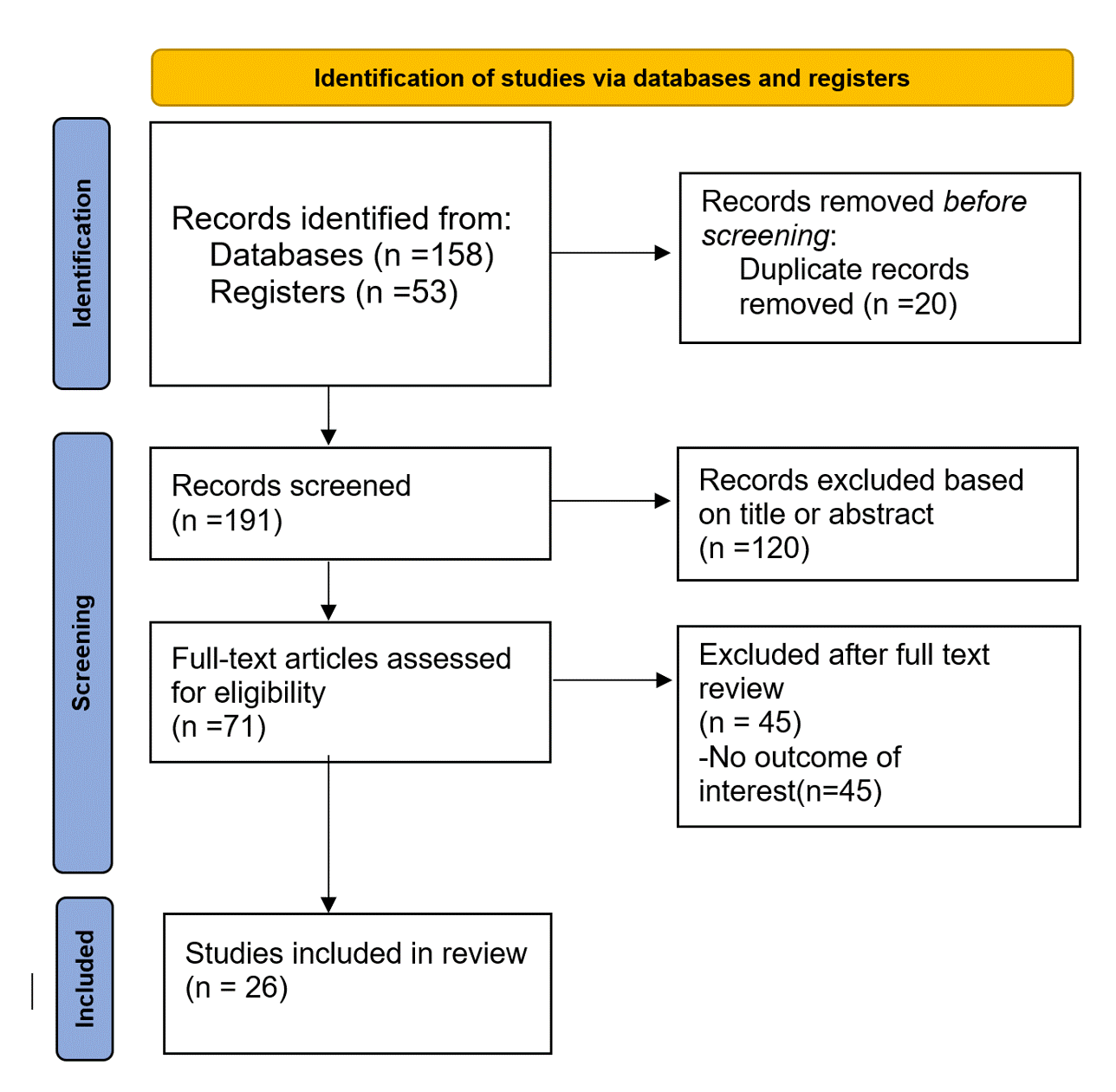
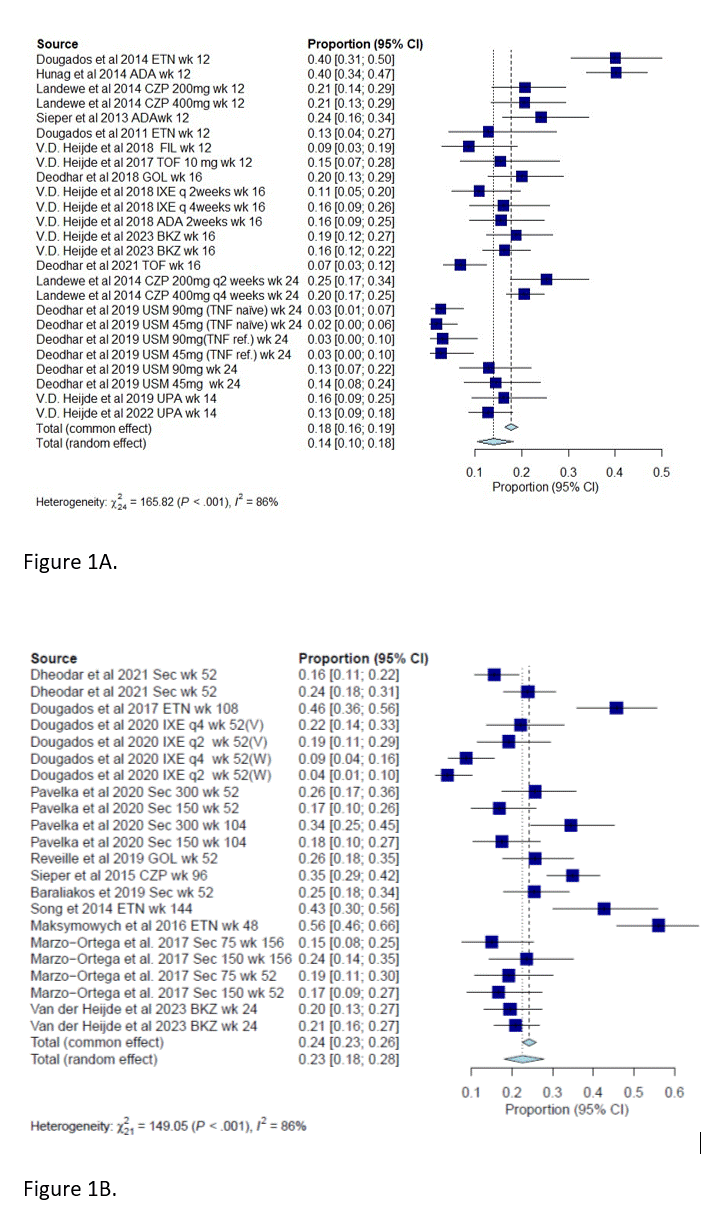
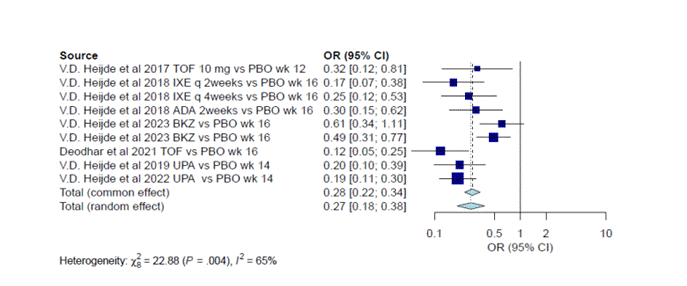
Results: Twenty-six clinic trials (randomized and open-label extensions) were identified (PRISMA flow chart attached) with 5024 patients included in the meta-analysis. The proportion of patients reaching inactive disease status in the treatment group at 12-24 weeks was 18% (95% CI:0.16-0.19), and at 52-104 was 24% (95% CI: 0.23-0.26). (Figures 1A and 1B). The odds of achieving inactive disease was 0.28 compared to low disease activity in the treatment group (95% CI 0.22-0.34) (Figure 2A)
Conclusion: The proportion of patients with axSpA achieving inactive disease status was low, suggesting that achieving inactive disease status may be an overarching goal. Hence the definition of clinical remission may need to be revisited with a more feasible outcome.
Figure 1B. Overall rate of inactive disease in the intervention group in studies ranging from 52_104 weeks
To cite this abstract in AMA style:
Abi Doumeth S, Pamuk O, Magrey M. Achieving ASDAS Inactive Disease Status in Axial Spondyloarthritis: A Systematic Review and Meta-analysis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/achieving-asdas-inactive-disease-status-in-axial-spondyloarthritis-a-systematic-review-and-meta-analysis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/achieving-asdas-inactive-disease-status-in-axial-spondyloarthritis-a-systematic-review-and-meta-analysis/