Session Information
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: RNA-immunoprecipitation (RNA-IP) is considered the gold standard method for anti-tRNA synthetase antibodies (ARS-Ab) detection. This technique, however, is not widely available, unlike commercial kits, whose performance, though, is often suboptimal. We performed centralized RNA-IP on serum samples of patients with anti-synthetase syndrome (ASSD) or other autoimmune diseases from 11 centers around the world, to determine the performance of local commercial testing compared to centralized RNA-IP in the classification criteria of ASSD (CLASS) project
Methods: Collected serum samples were tested centrally by one of the authors with proven experience in RNA-IP following a previously described protocol. The samples were randomly collected form patients diagnosed with ASSD (cases) or with other autoimmune diseases (controls). The author performing RNA-IP was blinded for the local diagnosis or autoantibody results. Results were provided as presence/absence of ARS-Ab (ARS-Ab+/ARS-Ab-). In patients with positive ARS-Ab by RNA-IP, anti-Jo1 specificity was tested through validated ELISA kit. The results of local testing were then compared to the central RNA-IP, using sensitivity, specificity, predictive values and overall accuracy
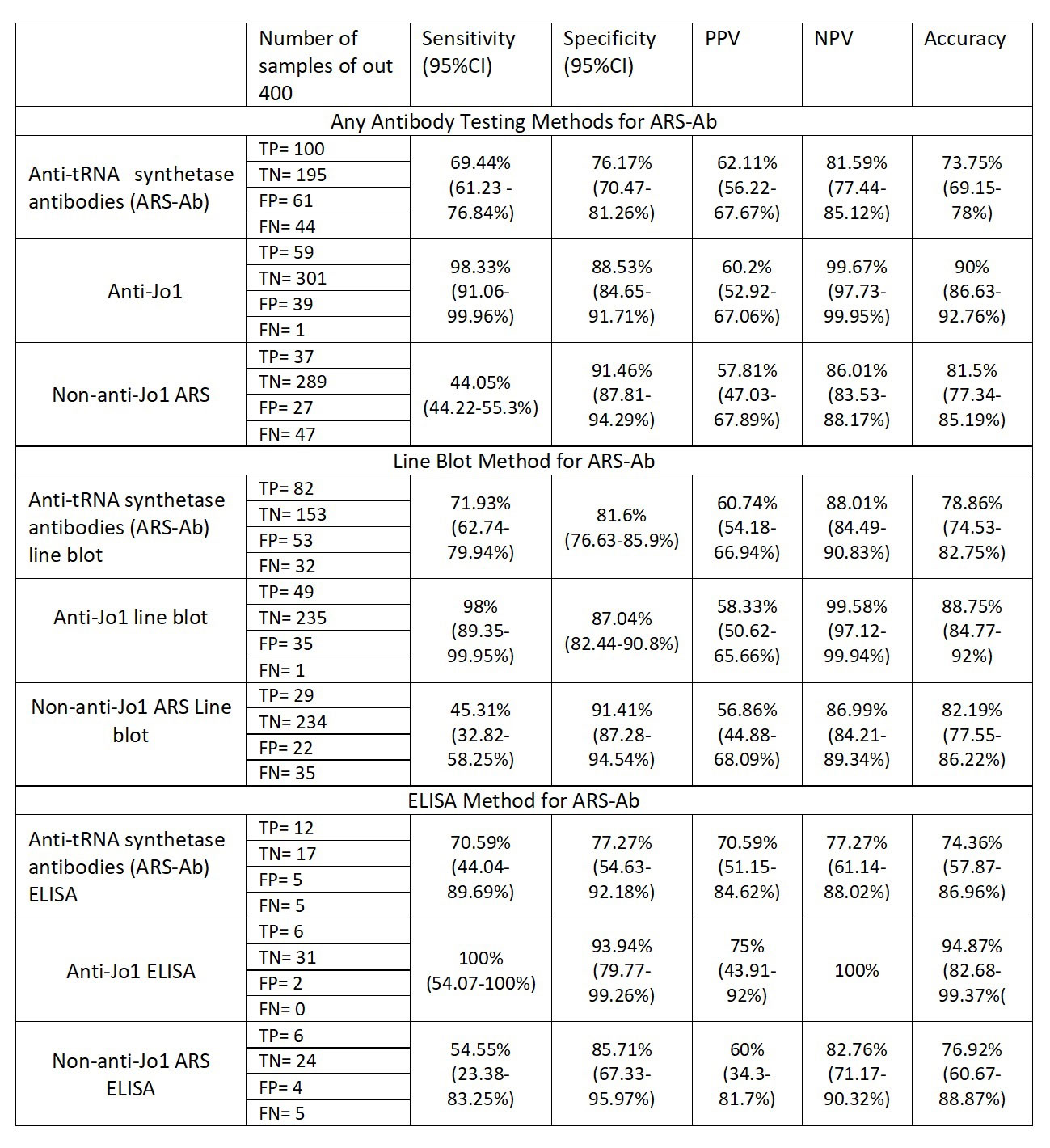
Results: Complete data about local autoantibody testing were available for 400 samples, 164 cases (41%) and 236 controls (59%) as per local diagnosis. Overall, 161 samples were ARS-Ab+ according to local determination (Local ARS-Ab+), 98 for anti-Jo1 and 63 for non-anti-Jo1, whereas 144 samples were ARS-Ab+ in central RNA-IP (central IP ARS-Ab+), 60 for anti-Jo1 and 84 for non-anti-Jo1. Local testing methods were line-blot (320 samples, 80%), ELISA (39, 9.75%) and RNA-IP (7, 1.75%).
Among the cases, 156 were Local ARS-Ab+ (95.1%), 96 for anti-Jo1 (58.5%) and 60 for non-anti-Jo1 ARS-Ab (36.6%). When tested centrally, only 101 were ARS-Ab+ (61.6%), 58 for anti-Jo1 (35.4%) and 43 for non-anti-Jo1 (26.2%). Among the controls, Local ARS-Ab+ were 5 (2.1%), 2 anti-Jo1 (0.8%) and 3 non-anti-Jo1 (1.3%), whereas central IP ARS-Ab+ were 43 (18.2%), 2 anti-Jo1 (0.8%) and 41 non-anti-Jo1 (17.4%) (Table1).
Considering the central IP as the gold standard, the overall sensitivity and specificity of local antibodies determination was calculated as 69.4% and 76.17%, respectively. When considering only anti-Jo1, the sensitivity and specificity were 98.33% and 88.53%, while for non-anti-Jo1 ARS-Ab they were 44.05% and 91.46%%, respectively. When considering only line-blot, the sensitivity was 71.93% and the specificity was 81.6% for all ARS-Ab, while for anti-Jo1 sensitivity was 98% and specificity was 87.04% and for non-anti-Jo1 sensitivity was 45.31% and specificity was 91.41%. ELISA showed better sensitivity than line-blot especially amongst non-Jo1- ARS-Ab patients (Table2)
Conclusion: These results show that local commercial testing of ARS-Ab is affected by a high rate of false positive and false negative results, especially for non-anti-Jo1 ARS-Ab leading to lower sensitivity and PPV. Most discrepancies were seen in line blot assay. It could be speculated that the local diagnosis was highly influenced by the results of local ARS-Ab testing, thus potentially leading to a misdiagnosis
To cite this abstract in AMA style:
Zanframundo G, Ceribelli A, Faghihi Kashani S, Morosini M, Bozzini S, Isailovic N, Selmi C, Cavagna L, Aggarwal R. Accuracy of Multi-Centric Local Determination of Anti-Synthetase Autoantibodies in Patients with Anti-Synthetase Syndrome and Other Autoimmune Diseases [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/accuracy-of-multi-centric-local-determination-of-anti-synthetase-autoantibodies-in-patients-with-anti-synthetase-syndrome-and-other-autoimmune-diseases/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/accuracy-of-multi-centric-local-determination-of-anti-synthetase-autoantibodies-in-patients-with-anti-synthetase-syndrome-and-other-autoimmune-diseases/