Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Behçet’s disease (BD) is a chronic vasculitis characterized by polymorphonuclear neutrophils (PMNs) hyperactivation with unknown etiology. The over-productions of neutrophil extracellular traps (NETs) and pro-inflammatory cytokines are increasingly implicated in BD (Clin Immunol. 2023; 250:109318). Aberrant metabolic intermediates have been identified as endogenous danger signals to elicit natural immune responses (Trends Endocrinol Metab. 2020; 31:712-724), yet relevant mechanisms in BD remained unexplored. Farnesyl pyrophosphate (FPP) is a key isoprenoid intermediate in the mevalonate (MVA) pathway that triggers pro-inflammatory responses (Sci China Life Sci. 2015; 58:328-35) and cell death (PLoS Biol. 2021;19:e3001134). Thus, we aimed to reveal the immunometabolic aberrations of BD, and highlight the pathogenic implications of FPP in BD-PMN hyperactivation.
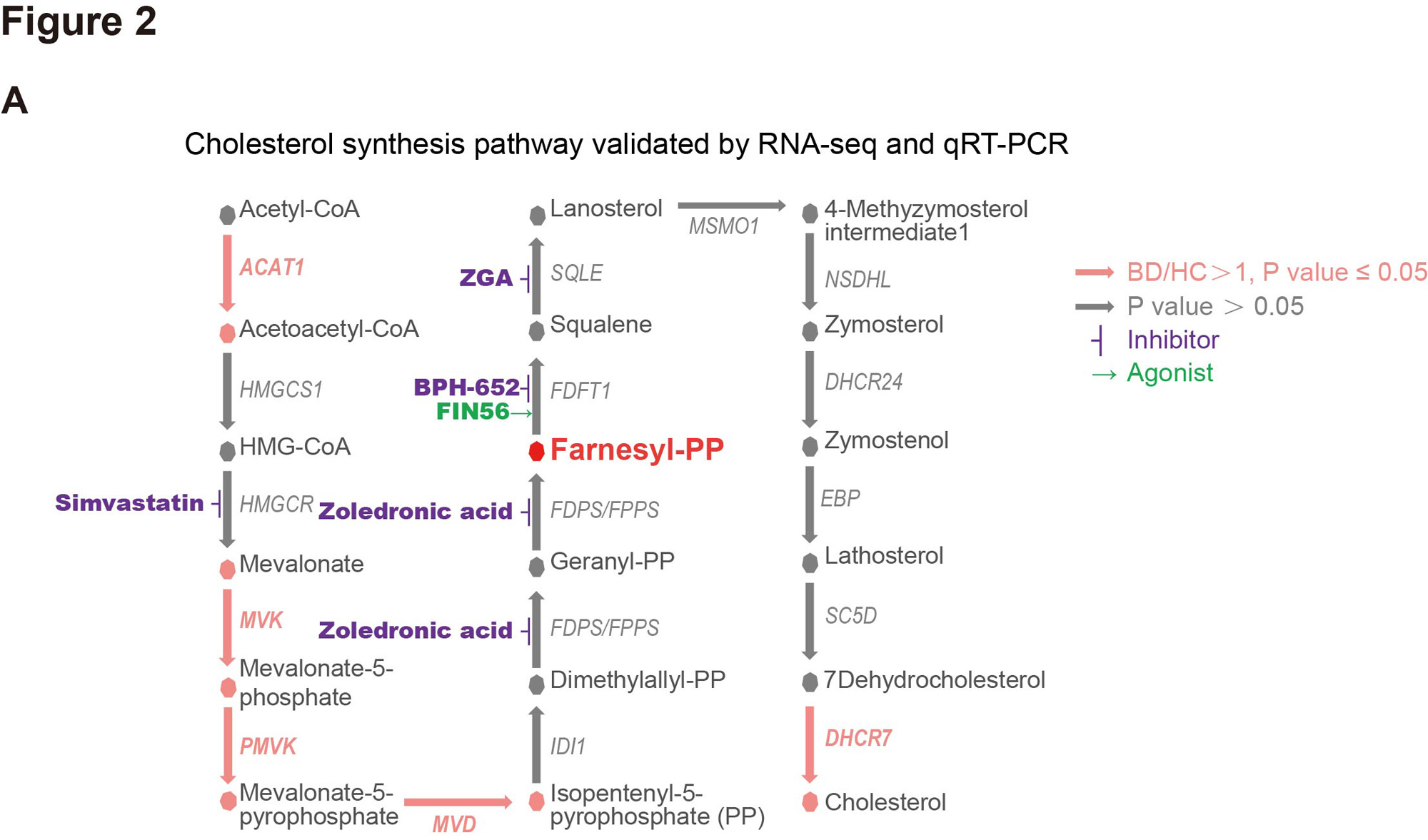
Methods: Focusing on immunometabolism, we integrated and analyzed our published data of serum lipidomics and peripheral blood immunocyte transcriptomics. Transcriptomic analysis and qRT-PCR validated the enhanced MVA pathway and suggested its metabolite FPP accumulated in BD-PMN.To elucidate the involvement of the MVA pathway in BD-PMN hyperactivation, we intervened with its key enzymes using appropriate inhibitors and agonists (Fig 2). We assayed the level of FPP in serum and PMN from BD and HC by targeted mass spectrum, and clinical correlations by Pearson correlation analysis. We stimulated PMNs with FPP, and measured their production of pro-inflammatory cytokines by qRT-PCR and NETs by immunofluorescence. Then, we stimulated vascular endothelial cells (VECs) with the supernatant from FPP-stimulated PMNs and measured the expression of activation/injury markers in the VECs by qRT-PCR.
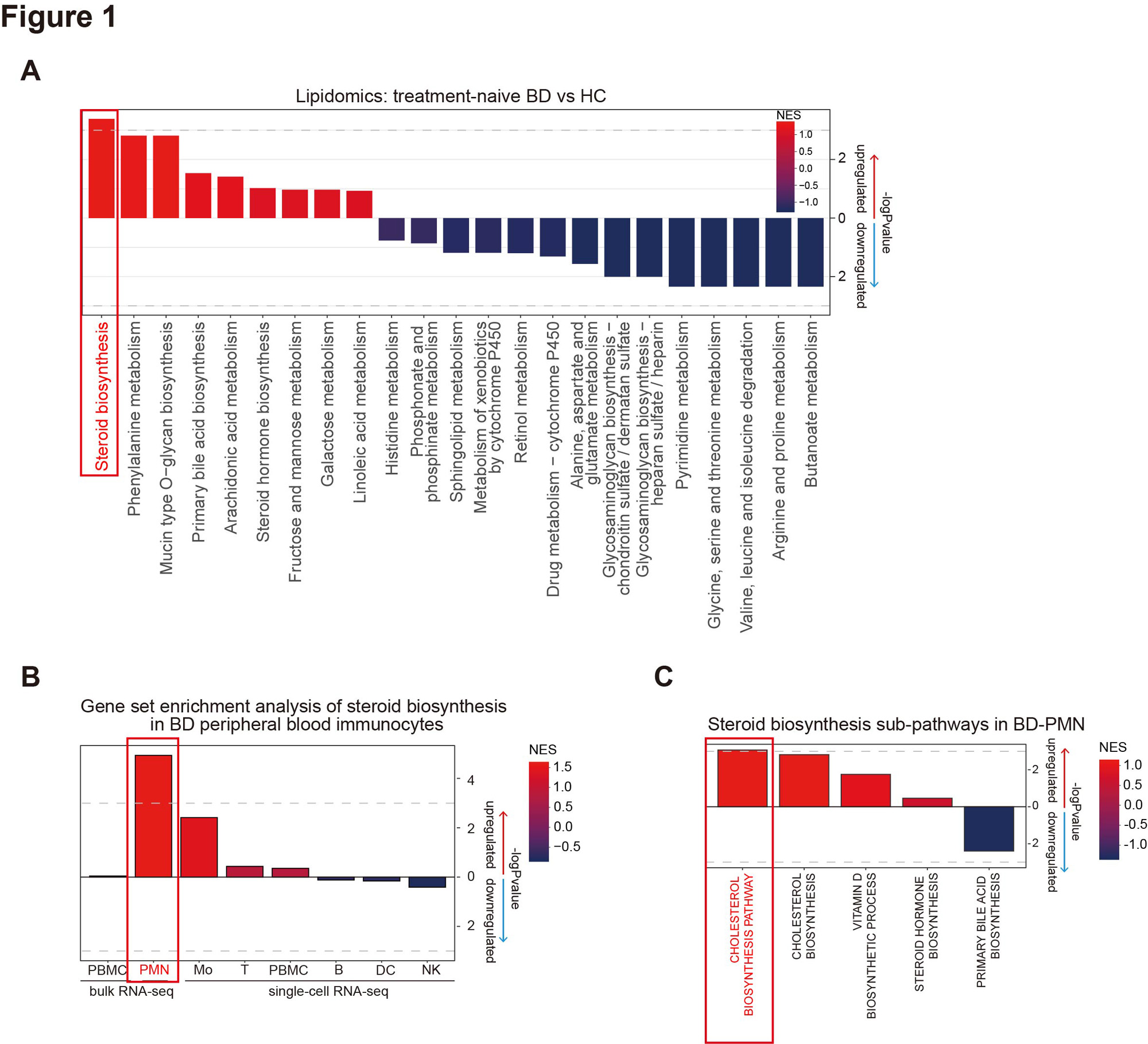
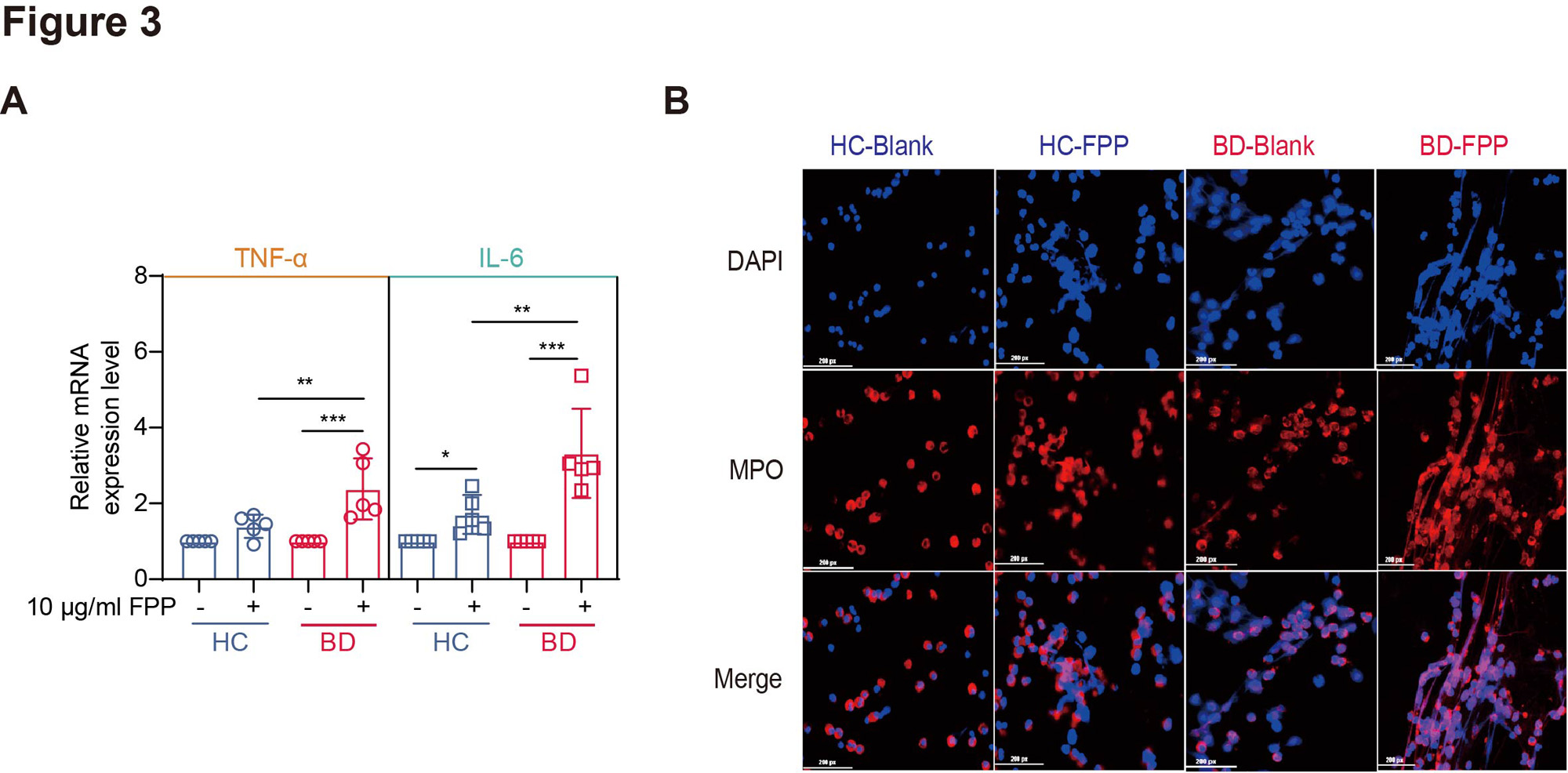
Results: Our multi-omics analysis revealed that steroid biosynthesis was significantly elevated in BD serum, with consistent metabolic aberration noted only in PMN (Fig 1A-1B), particularly in cholesterol biosynthesis (Fig 1C). More precisely, the upregulation of enzymes upstream of FPP suggested the enhanced MVA pathway and accumulated intermediate FPP (Fig 2). Furthermore, FPP was confirmed to be the key component of the MVA pathway promoting PMN activation, as its inhibition decreased PMN cytokine productions (zoledronic acid, p< 0.0001), while its metabolism inhibition had facilitative effects (BPH-652, p< 0.0001), and vice versa (FIN56, p< 0.0001). FPP was overexpressed in BD serum (p=0.0022) and PMN (p=0.0397), and positively correlated with BD disease activity. Notably, FPP specifically promoted PMN to produce proinflammatory cytokines and NETs in a dose-dependent manner, further damaging VECs and leading to the overexpression of molecules related to inflammation (TNF-α, p< 0.0001), adhesion (ICAM-1, p=0.0006), and permeability (VEGF, p=0.0004). And BD-PMN was hyper-responsive to FPP (Fig 3).
Conclusion: Overall, our study revealed the aberrant immunometabolic profiles of BD and highlighted the effect of FPPon promoting PMN hyperactivation, providing insight into the potential of targeting FPP for BD treatment. Further investigations are in progress to elucidate the mechanisms.
A: Lipidomic analysis between treatment-naïve BD and HC serum. B: Gene Set Enrichment Analysis (GSEA) of steroid biosynthesis pathway in BD and HC peripheral blood immunocytes, including bulk RNA-seq of PBMC (GSE198533), PMN (GSE205867) and single-cell RNA-seq of PBMC (GSE198616). C: GSEA of steroid synthesis sub-pathways, such as bile acids, steroid hormones, vitamin D, and cholesterol synthesis, in BD and HC PMN (GSE205867).
A: A schematic diagram of the cholesterol biosynthesis pathway. Enzymes with significantly elevated expression levels in BD-PMN, as verified by both RNAseq and qRT-PCR (N = 20), were marked in red. Potential agonists and inhibitors were highlighted with green and purple arrows, respectively.
A: qRT-PCR measurement of TNF-α and IL-6 expression levels in PMNs from BD (red), and HC (blue), after stimulation with 10 μg/ml FPP (N=5). *, p<0.05; **, p<0.01; ***, p<0.001; B: Representative immunofluorescence staining diagrams of 60 μg/ml FPP-induced NETosis in PMNs from BD and HC, showing MPO (red) and DAPI-stained nuclei (blue); scale bar = 50 μm. MPO: Myeloperoxidase.
To cite this abstract in AMA style:
Zhang M, Kang N, Yu X, Liu W, Zheng W. Aberrant Mevalonate Metabolite Farnesyl Pyrophosphate-Induced Neutrophil Hyperactivation in Behçet’s Disease Pathogenesis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/aberrant-mevalonate-metabolite-farnesyl-pyrophosphate-induced-neutrophil-hyperactivation-in-behcets-disease-pathogenesis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/aberrant-mevalonate-metabolite-farnesyl-pyrophosphate-induced-neutrophil-hyperactivation-in-behcets-disease-pathogenesis/