Session Information
Date: Sunday, November 13, 2022
Title: SLE – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
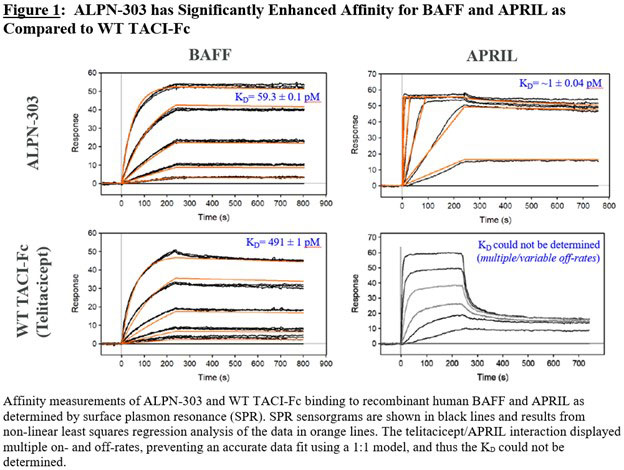
Background/Purpose: B cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL) are tumor necrosis factor (TNF) superfamily members that bind TACI (transmembrane activator and CAML interactor), BCMA (B cell maturation antigen), and/or BAFF-R on B cells and together support B cell development, differentiation, and survival. Therapeutic agents targeting BAFF and/or APRIL, including the wild-type (WT) TACI-Fc fusion proteins atacicept and telitacicept, have demonstrated promising clinical potential in rheumatic diseases like systemic lupus erythematosus (SLE) and other B-cell-related diseases such as autoantibody-related nephritides; however, there is still need for more efficacious yet safe therapies. ALPN-303 is an Fc fusion of an engineered TACI variant TNFRSF domain (vTD) which substantially improves upon the ligand affinity liabilities of WT TACI (Figure 1), resulting in highly potent dual BAFF/APRIL inhibition superior to WT TACI-Fc, or BAFF- or APRIL-specific mAbs. In preclinical studies, ALPN-303 demonstrated enhanced pharmacokinetic (PK) and immunomodulatory properties vs. WT TACI-Fc, which may translate to lower and/or less frequent doses in humans. ALPN-303 also suppressed autoantibodies, renal IgG deposition, and nephritis in mouse models of lupus. ALPN-303 may therefore significantly improve clinical outcomes in SLE and other B-cell-related diseases.
Methods: In this first-in-human study (NCT05034484), healthy adult volunteers have been enrolled in single ascending dose cohorts of intravenously (IV) or subcutaneously (SC) administered ALPN-303. For each IV cohort, the first 2 sentinel subjects are randomized 1:1 to receive ALPN-303 or placebo, followed by the remaining 4 subjects randomized 3:1 to receive ALPN-303 or placebo. SC cohorts are randomized 4:2 to receive a single SC dose of ALPN-303 or placebo. All subjects are followed to assess safety, PK, circulating immunoglobulins (Ig), and peripheral B-cell populations.
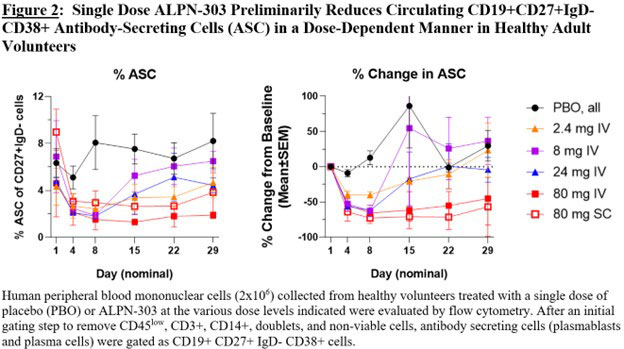
Results: ALPN-303 has been well tolerated in all cohorts evaluated to date and exhibits dose-dependent PK and expected pharmacodynamic (PD) effects on circulating Ig levels and B-cell populations, including dose-dependent reductions in circulating CD38+ (CD19+CD27+IgD-) antibody-secreting cells (Figure 2) and in serum immunoglobulin (Figure 3). To date, there have been no serious adverse events, no infusion-related or injection site reactions, and no adverse trends in safety laboratories. Dose escalation will be complete by the time of the meeting; the presentation will include all available safety, PK, and PD data.
Conclusion: In this first-in-human study, ALPN-303 demonstrates acceptable preliminary safety and tolerability and exhibits dose-dependent PK and expected PD effects on circulating Ig and B-cell populations. These findings support future clinical development of ALPN-303 in patients with SLE or other B-cell- and/or autoantibody-related diseases.
To cite this abstract in AMA style:
Dillon S, Harrison P, Lickliter J, Manjarrez K, Smith A, Lessig M, Blanchfield L, Sanderson R, Chunyk A, Blair T, Enstrom A, Wolfson M, Rixon M, Zayed H, Davies R, Peng S. A Randomized Placebo-Controlled Phase 1 Study in Healthy Adult Volunteers of the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of ALPN-303, a Potent Dual BAFF/APRIL Antagonist for the Treatment of Systemic Lupus Erythematosus and Other Autoantibody-Associated Diseases [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/a-randomized-placebo-controlled-phase-1-study-in-healthy-adult-volunteers-of-the-safety-tolerability-pharmacokinetics-and-pharmacodynamics-of-alpn-303-a-potent-dual-baff-april-antagonist-for-the-t/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-randomized-placebo-controlled-phase-1-study-in-healthy-adult-volunteers-of-the-safety-tolerability-pharmacokinetics-and-pharmacodynamics-of-alpn-303-a-potent-dual-baff-april-antagonist-for-the-t/