Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: FX006 is a novel sustained-release formulation of TCA in poly(lactic-co-glycolic) acid microspheres intended to maintain therapeutic concentrations in the joint up to 3 months following IA injection. In patients (pts) with OA of the knee, FX006 was compared to an approved injectable suspension of TCA (TCA IR) to identify a dose of FX006 to move forward into Phase 3.
Methods: Pts with baseline average daily pain intensity (ADP) of ≥5 to ≤9 on an 11 point Numeric Rating Scale (NRS) ranging from 0 (no pain) to 10 (pain as bad as you can imagine) were randomized (1:1:1:1) and treated with a single IA injection of FX006 (containing 10, 40 or 60 mg of TCA) or 40 mg of TCA IR. Pts were evaluated at 7 visits over 12 wks for efficacy, safety and PK. Primary endpoint: change from Baseline to each of Wks 8, 10 and 12 in the weekly mean of ADP. Secondary endpoints: time to onset of pain relief, WOMAC (pain, stiffness and function), responder status, and global impression of change.
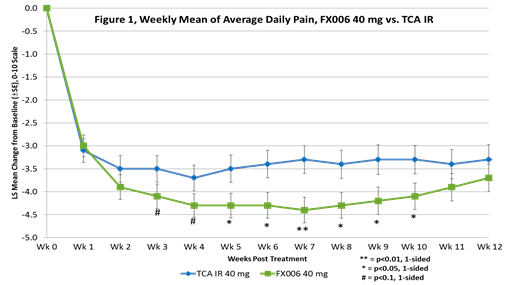
Results: Pts (228) were randomized and treated (FX006 10 mg, n=58, FX006 40 mg, n=59, FX006 60 mg, n=60; TCA IR, n=51). Treatment arms were well balanced across demographic and baseline characteristics (mean baseline ADP: 6.5). FX006 40 mg was superior to TCA IR on weekly mean of ADP at Wk 5 to Wk 10 (p<0.05) (average pain reduction -0.9) (Figure 1). The 40 mg dose also demonstrated significant improvement over TCA IR (p<0.05) in secondary outcomes at Wk 8 (Table 1). Time to onset of pain relief was the same for FX006 40 mg and TCA IR. The 10 mg dose produced effects that were consistently improved relative to TCA IR but of lessor magnitude than those of the 40 mg dose. The 60 mg dose did not produce an improvement relative to the 40 mg dose. There were no related SAEs. AEs were generally mild and unrelated to study drug. Local knee-related AEs, lab assessments, ECGs and vital signs were unremarkable and similar across all treatments. Plasma PK across the FX006 doses were linear and dose-dependent. Peak plasma concentrations achieved with TCA IR were 10x of that seen with FX006 40 mg.
Conclusion: In pts with OA of the knee, the 40 mg dose of FX006 demonstrated an amplified and prolonged therapeutic effect relative to TCA IR. A Phase 3 study of the 40 mg dose of FX006 is being planned.
Disclosure:
N. Bodick,
Flexion Therapeutics,
1,
Flexion Therapeutics,
3;
J. Lufkin,
Flexion Therapeutics,
1,
Flexion Therapeutics,
3;
C. Willwerth,
Flexion Therapeutics,
1,
Flexion Therapeutics,
3;
P. Lachance,
None;
G. Jasey,
None;
A. Gupta,
None;
A. Chris,
None;
M. Russo,
None;
M. O’Mahony,
None;
S. Henein,
None;
L. Murdoch,
None;
F. de Looze,
None;
D. Hunter,
None;
M. Clayman,
Flexion Therapeutics,
1,
Flexion Therapeutics,
3.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-randomized-double-blind-dose-ranging-study-comparing-fx006-an-intra-articular-ia-sustained-release-formulation-of-triamcinolone-acetonide-tca-to-an-approved-injectable-suspension-of-tca-in-p/