Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Utilizing Patient-Reported Outcomes Measurement Information System (PROMIS®) questionnaires can enhance clinical care by measuring longitudinal changes in symptom severity as reported by the patient. This pilot aimed to assess the feasibility and impact of incorporating PROMIS® questionnaires at the point of care in rheumatology practice.
Methods: Patients with rheumatic diseases and decrements in ≥1 PROMIS® domain (pain intensity, physical function, or sleep disturbance) were stratified by their concerning domain, then randomized to either receive an interpretation of their PROMIS® scores before their rheumatology appointment (Arm 1) or to usual care (Arm 2) (ClinicalTrials.gov ID: NCT05026853). The primary outcome was the documentation of PROMIS® scores in the electronic medical record (EMR). Secondary outcomes include recommendations made by physicians based on PROMIS® scores, patient-provider communication, and change in the most concerning PROMIS® domain score from baseline to 12 weeks.
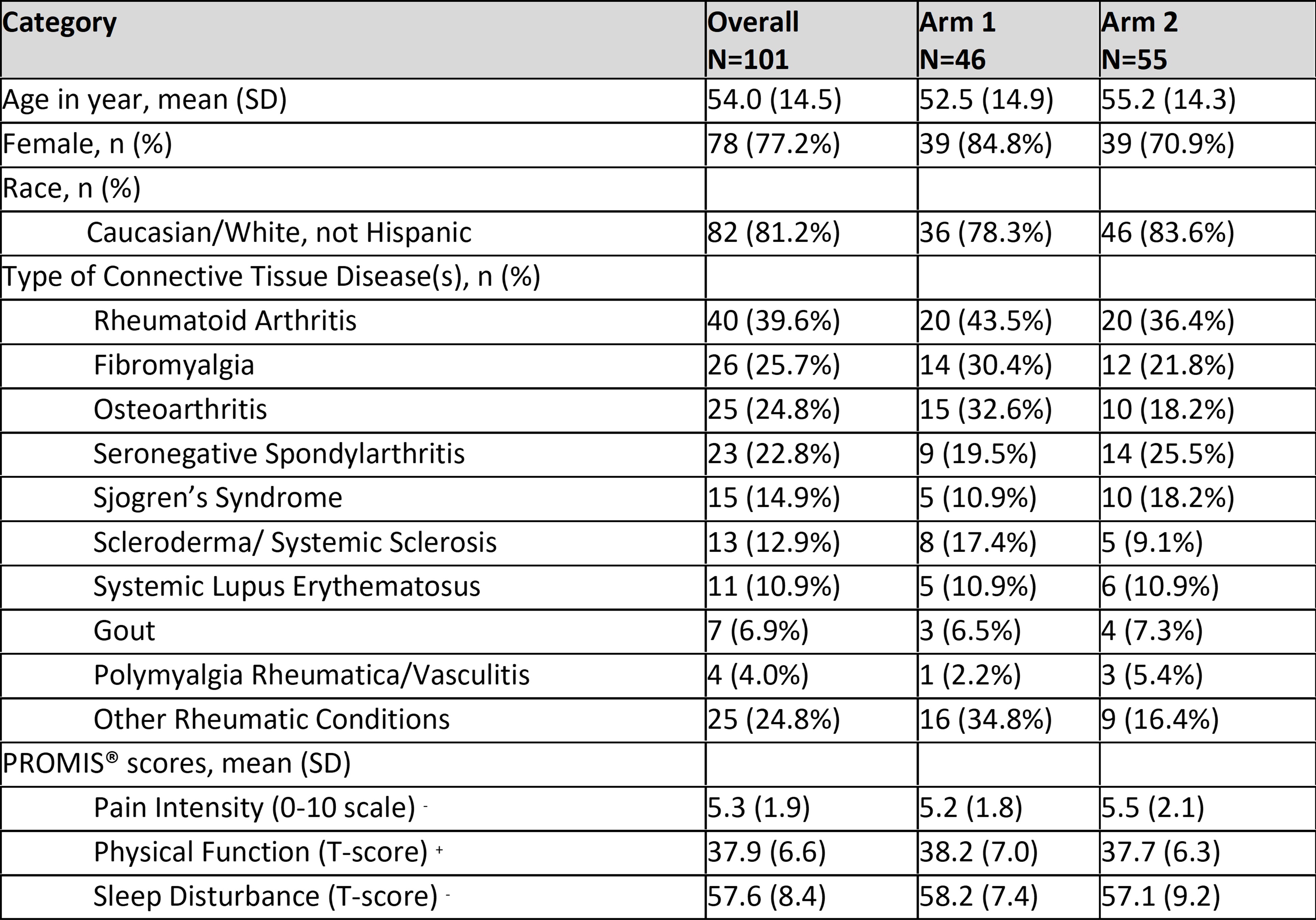
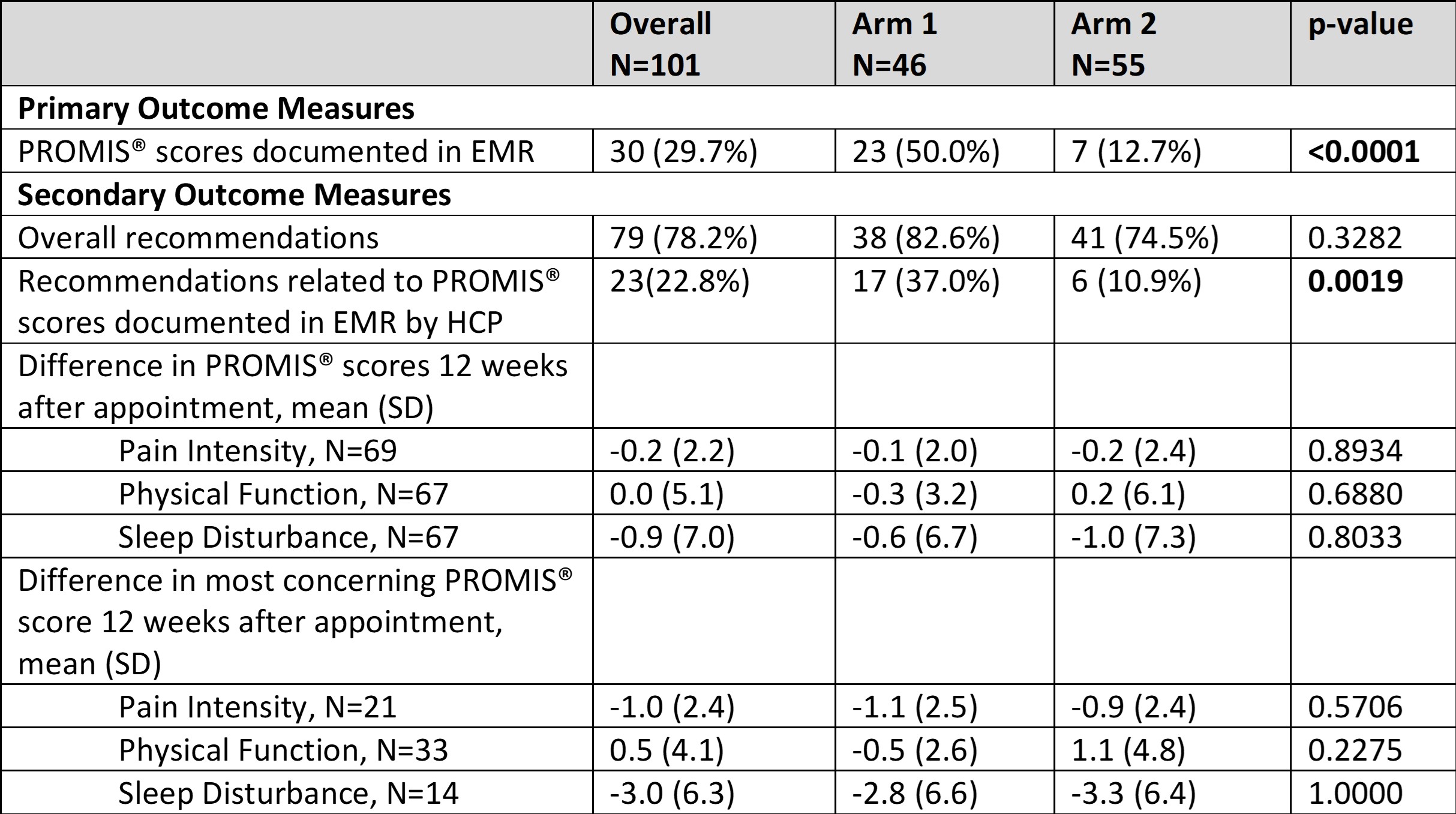
Results: 110 patients were enrolled. 55 were randomized to receive report cards (Arm 1), of which 46 received the report card, and 55 received usual care (Arm 2). Subjects had various rheumatologic conditions as described in Table 1. Documentation of PROMIS® scores in the EMR was present for 50% of subjects in Arm 1 (12.7% in Arm 2, p< 0.0001, Table 2). More recommendations were made based on PROMIS® scores for Arm 1 patients. There was no significant difference in post-visit PROMIS® score improvement between Arm 1 and Arm 2.
Conclusion: Providing PROMIS® report cards to patients and healthcare providers increased score documentation in the EMR. Increased recommendations made based on PROMIS® scores in Arm 1 suggest that having a score interpretation might help direct medical decision-making. PRO implementation could have various effects such as influencing patient health behaviors, detecting unrecognized problems, supporting discussions of PROs between patients and HCPs, and aiding HCPs in monitoring patients’ health.1 These factors contribute to the intermediate processes that occur before we can see improvement in the distal outcome of patient health status or quality of life. Our results demonstrate an improvement in the documentation of PRO scores in the EMR is a positive proximal outcome. This is a positive and necessary step to reach the distal outcome of improving patients’ outcomes.
References:
1. Khanna, Dinesh et al. “The future of measuring patient-reported outcomes in rheumatology: Patient-Reported Outcomes Measurement Information System (PROMIS).” Arthritis care & research vol. 63 Suppl 11,Suppl 11 (2011): S486-90. doi:10.1002/acr.20581
Some patients advocated >1 diagnosis.
+ indicates higher score is better, – indicates lower score is better.
To cite this abstract in AMA style:
Gedert R, Ochocki D, Kortam N, Huang S, Nagaraja V, Chakrabarti K, Ford J, Garber M, Lee J, Ognenovski V, Roofeh D, Cella D, Khanna D. A Pilot Trial of Integrating Patient-Reported Outcome Measurement Information System (PROMIS®) into Rheumatology Care [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/a-pilot-trial-of-integrating-patient-reported-outcome-measurement-information-system-promis-into-rheumatology-care/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-pilot-trial-of-integrating-patient-reported-outcome-measurement-information-system-promis-into-rheumatology-care/