Session Information
Session Type: Late-Breaking Abstract Session
Session Time: 9:00AM-10:30AM
Background/Purpose: Peresolimab is a humanized immunoglobulin G1 monoclonal antibody that stimulates human programmed cell death protein 1 (PD-1). We hypothesized that peresolimab binding to PD-1, a checkpoint inhibitory receptor, could stimulate physiological immune inhibitory pathways to restore immune homeostasis; this represents a novel approach to treating patients with autoimmune or autoinflammatory diseases.
Methods: This abstract reports results from a Phase 2a, placebo-controlled, double-blind, randomized clinical trial (NCT04634253) evaluating the efficacy and safety of peresolimab in adult participants with moderately to severely active rheumatoid arthritis (RA) who have had an inadequate response to prior disease modifying drugs, either conventional (csDMARDs), biologic (bDMARDs) or synthetic (tsDMARDS).
Treatment comparisons versus placebo were made using mixed effects model for repeated measures (MMRM) and using logistic regression model for continuous and binary endpoints, respectively. Nominal p values are reported. Missing data for binary endpoints were imputed as non-response.
Results: One hundred and one patients were randomly assigned 2:1:1 to receive intravenous peresolimab 700 mg (n = 49), 300 mg (n = 25), or placebo (n = 24) Q4W; 98 participants received at least one dose of study treatment and were included in the analysis.
Baseline demographics and disease activity were similar among groups. The majority (83.7%) of participants were female, and the mean (SD) age at baseline was 51.7 (12.6) years. At baseline, the mean (SD) duration of RA was 10.0 (8.0) years, and the mean (SD) DAS28-CRP score was 5.9 (0.85).
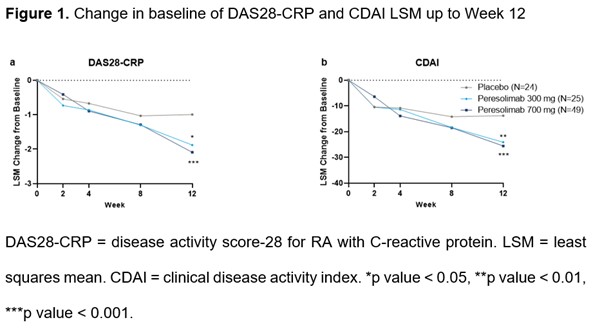
This trial met its primary endpoint of a significantly greater improvement from baseline at Week 12 in DAS28-CRP score in participants treated with peresolimab vs participants treated with placebo at both tested doses (700 mg [p < 0.001] and 300 mg [p = 0.017], figure 1a).
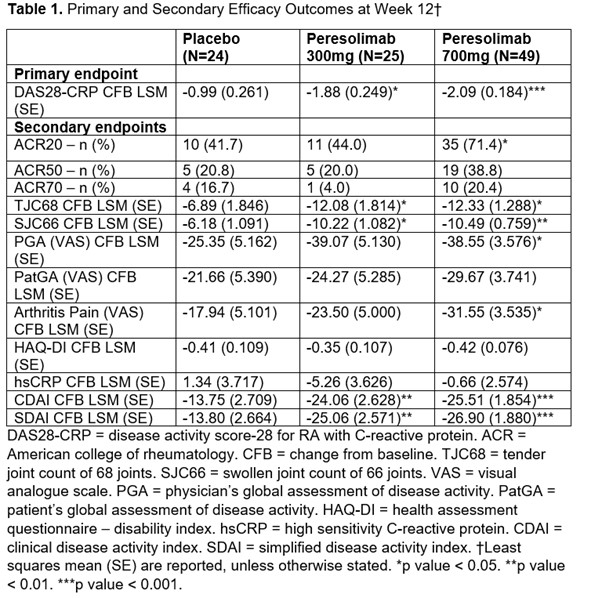
Significant improvements were also seen in CDAI between participants treated with either peresolimab dose (300 mg [p = 0.008] and 700 mg [p < 0.001], figure 1b) and placebo, and a significantly greater percentage of participants treated with peresolimab 700 mg achieved ACR20 (p < 0.05) compared to participants treated with placebo by Week 12 (table 1).
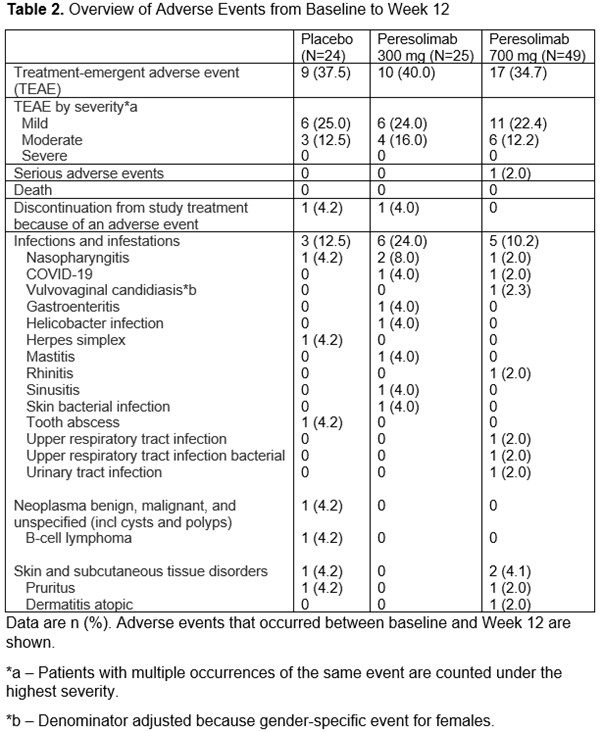
Furthermore, efficacy was maintained through Week 24 in patients achieving CDAI low disease activity at Week 14. Peresolimab exhibited a safety and tolerability profile that supports further clinical evaluation in immunologic disease.
Conclusion: These clinical data represent the first meaningful evidence that stimulating the PD-1 receptor has the potential to treat RA. Peresolimab was superior to placebo at Week 12 for several key endpoints. Safety events were similar between treatment groups. Future studies will continue evaluating peresolimab as treatment for RA, and for other autoimmune diseases.
To cite this abstract in AMA style:
Tuttle J, Drescher E, Simón-Campos J, Emery P, Greenwald M, Kivitz A, Rha H, Yachi P, Kiley C, Nirula A. A Phase 2 Trial of Peresolimab for Adults with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/a-phase-2-trial-of-peresolimab-for-adults-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-phase-2-trial-of-peresolimab-for-adults-with-rheumatoid-arthritis/